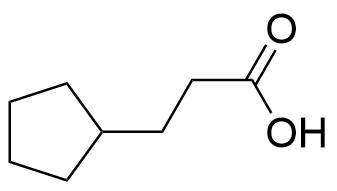
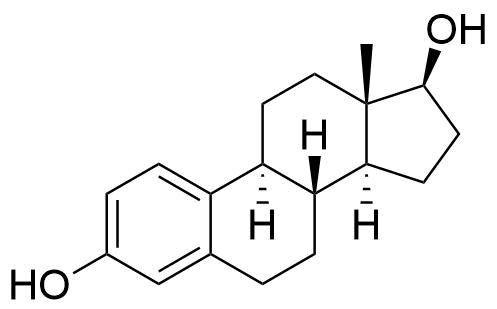
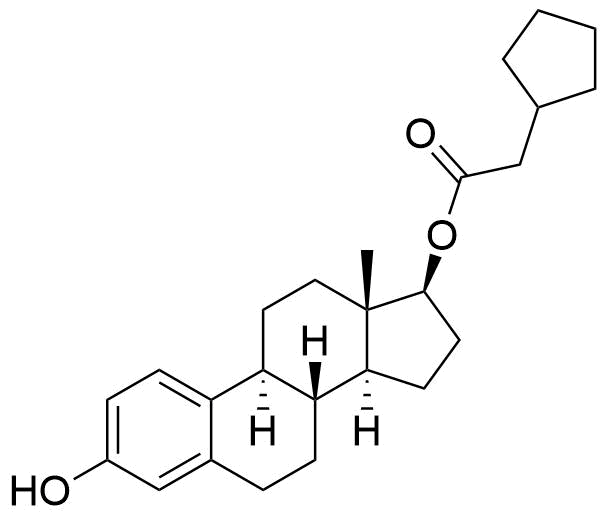
Estradiol Cypionate
Estradiol Cypionate (EV or E2V) is an ester prodrug of estradiol and is used in place of estradiol in some medications.
Tags
Approvals
WHO Essential Medicine US FDA-ApprovedRelated Compounds
Estradiol Estradiol Valerate
Identifiers
Abbreviation
EC, E2C
References
Names
- Estra-1,3,5(10)-triene-3,17β-diol 17β-cylcopentylpropionate
- estradiol 17β-cyclopentylpropionate
- estradiol 17β-cyclopentanepropionate
References
CASRN
313-06-4
References
PubChem CID
9403
ECHA InfoCard
- 100.005.672
- EC / List #: 206-237-8
DrugBank Accession Number
DBSALT000067
References
- DrugBank: Estradiol Cypionate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
7E1DV054LO
KEGG Entry Number
D04063
Wikipedia Entry Name
Estradiol Cypionate
ChEBI ID
CHEBI:34745
ChEMBL ID
CHEMBL1200973
ChemSpider ID
9033
NIST
Estradiol Cypionate
Physical & Chemical Properties
Molecular Formula
C26H36O3
References
Molecular Weight
369.56 g/mol
References
Specific Optical Rotation
+39° to +44°, 20 mg/mL in dioxane
References
- USP 40: Estradiol Cypionate monograph. (View all citations for this reference)
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Carcinogenicity | 1B | H350 | May cause cancer |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
MRTD
0.5 mg/kg/day
Biochemistry & Pharmacology
Pharmacology
See estradiol
References
Estrogen Receptor Activity
Agonist
Metabolism
Metabolized by CYP3A4, CYP2C9, UGT
Enzyme Interactions
Inhibits CYP3A4, CYP2C9, CYP2B6 Induces UGTs
Metabolites
Impurities
Name
Structure
CASRN
Other Names & Identifiers
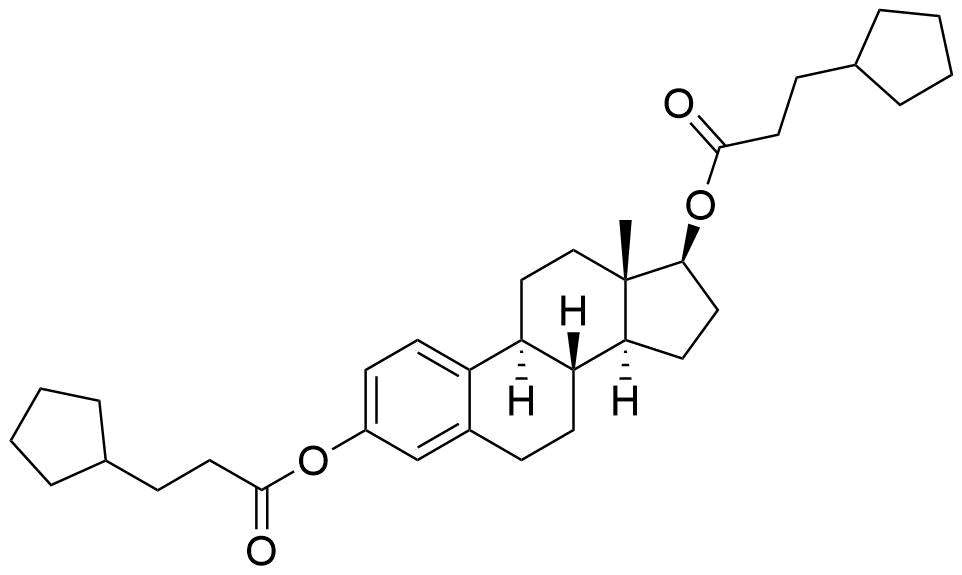
- BP Estradiol Cypionate Impurity D
- Estra-1,3,5(10)-trien-3,17β-diyl dicyclopentanepropanoate
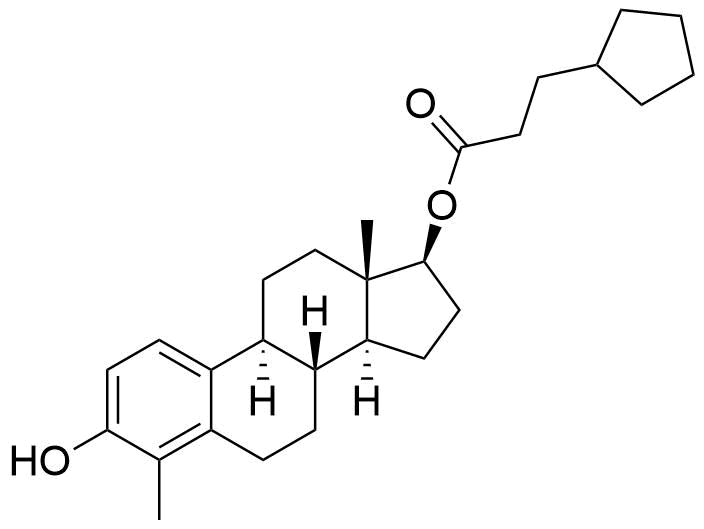
- BP Estradiol Cypionate Impurity C
- 3-hydroxy-4-methylestra-1,3,5(10)-trien-17β-yl cyclopentanepropanoate
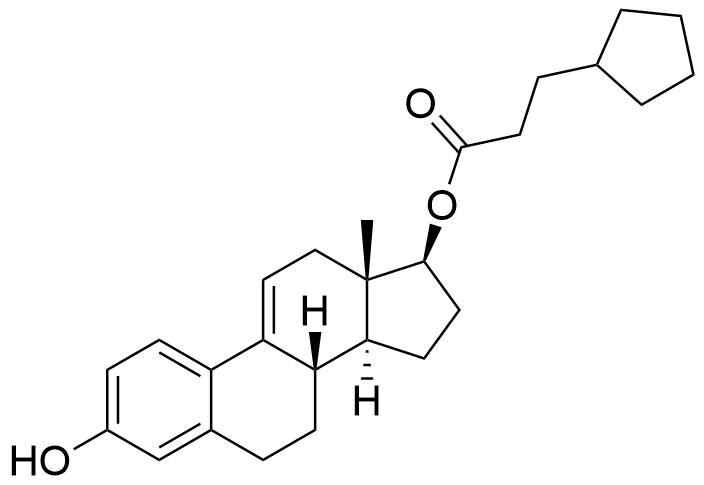
- BP Estradiol Cypionate Impurity B
- Δ9(11)-Estradiol Cypionate
- 3-hydroxyestra-1,3,5(10),9(11)-tetraen-17β-yl cyclopentanepropanoate
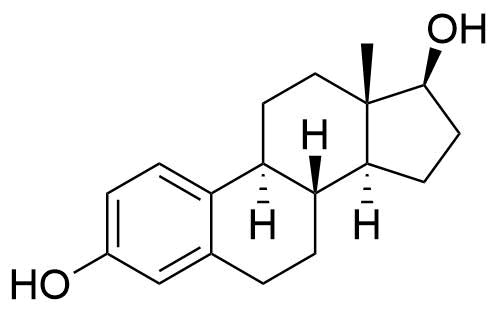
50-28-2
- BP Estradiol Cypionate Impurity A
- BP Estradiol Valerate Impurity A
- BP Ethinylestradiol Impurity D
- 17β-Estradiol
- Oestradiol
- Dihydrofolliculin
- Estrace
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Discontinued
085603
Discontinued
017968
Discontinued; Prescription
085470
WHO Essential Medicines
Name
Formulation
MEDROXYPROGESTERONE ACETATE: 25 mg
Injection