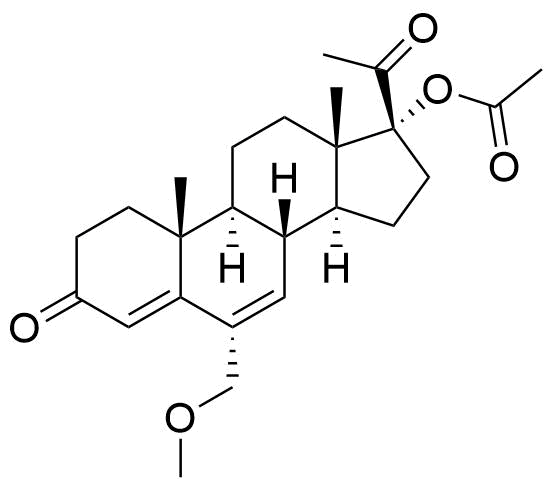
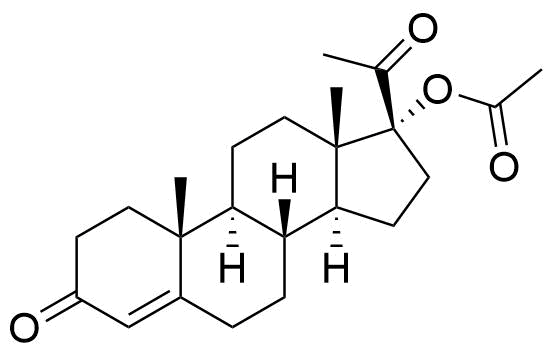
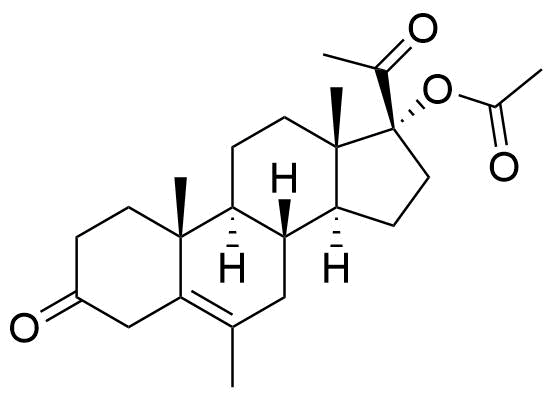
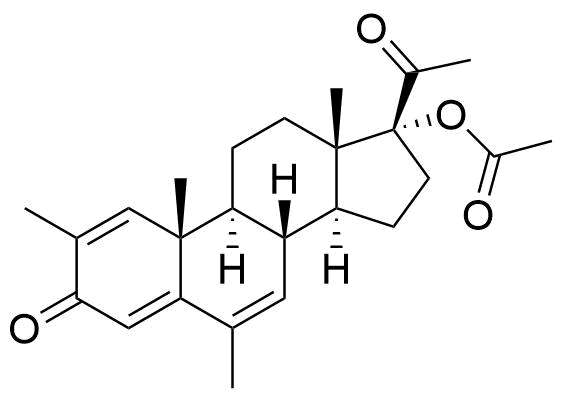
Megestrol Acetate
Megestrol Acetate (MGA) is a synthetic progestogen that was formerly used in combined oral contraceptives. It is currently used as an appetite stimulant and in the treatment of certain cancers.
Tags
Approvals
US FDA-Approved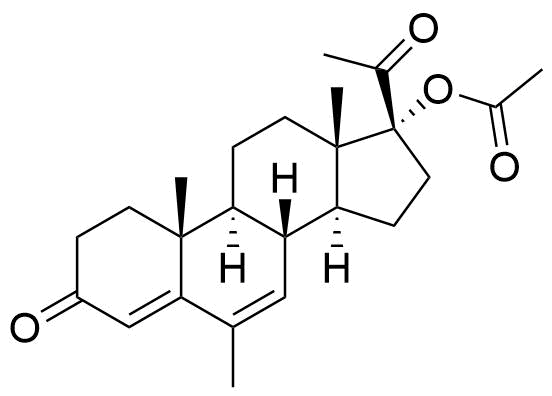
Identifiers
Abbreviation
MGA
References
Names
- USAN, INN, BAN, JAN: megestrol acetate
- 17α-acetoxy-6-dehydro-6-methylprogesterone
- 17α-acetoxy-6-methylpregna-4,6-diene-3,20-dione
- 6-methyl-3,20-dioxopregna-4,6-dien-17-yl acetate
References
CASRN
595-33-5
References
PubChem CID
11683
ECHA InfoCard
- 100.008.969
- EC / List #: 209-864-5
DrugBank Accession Number
DB00351
References
- DrugBank: Megestrol Acetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
TJ2M0FR8ES
Wikipedia Entry Name
Megestrol Acetate
ChemSpider ID
11192
NIST
Megestrol Acetate
ATC Code(s)
References
- DrugBank: Megestrol Acetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C24H32O4
References
Molecular Weight
384.5 g/mol
References
Appearance
White or almost white, odorless, crystalline powder
References
- British Pharmacopoeia 2017: Megestrol Acetate monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Melting Point
BP: About 217° C
ChemIDPlus: 214° C
References
- ChemIDPlus: A Toxnet Database. Megestrol Acetate. (View all citations for this reference)
- British Pharmacopoeia 2017: Megestrol Acetate monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Megestrol Acetate (View all citations for this reference)
Solubility
Practically insoluble in water (2 μg/mL at 37 °C). Very soluble in chloroform. Slightly soluble in diethyl ether and fixed oils. Soluble in acetone, sparingly soluble in ethanol (96%).
References
- ChemIDPlus: A Toxnet Database. Megestrol Acetate. (View all citations for this reference)
- British Pharmacopoeia 2017: Megestrol Acetate monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Specific Optical Rotation
+14.0 to +17.0, dried substance, 2.50 g in methylene chloride diluted to 25.0 mL
+5° in chloroform
References
- British Pharmacopoeia 2017: Megestrol Acetate monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Storage Conditions
Tablets should be stored in well-closed containers at <40 °C, recommended 15-30 °C. Oral suspension should be stored in tight containers <= 25 °C.
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Specific Target Organ Toxicity, Repeated Exposure | 2 | H373 | Causes damage to organs through prolonged or repeated exposure |
| Reproductive Toxicity | 1A | H360FD | May damage fertility. May damage the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Mutagenicity | 2 | H341 | Suspected of causing genetic defects |
| Carcinogenicity | 1B | H350 | May cause cancer |
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
LD50
mouse intravenous: 56 mg/kg
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist
References
Androgen Receptor Activity
Antagonist, weak partial agonist
Estrogen Receptor Activity
No activity
Glucocorticoid Receptor Activity
Agonist
Mineralocorticoid Receptor Activity
No activity at clinical doses.
Bioavailability
Has not been evaluated.
Elimination Half-Life (t1/2)
Mean 34.2 h, range 13-104.9 h
Metabolism
Mainly metabolized by CYP3A4, but also by CYP3A5. Hepatic.
References
- Seminerio, M. J.; House, L.; Mirkov, S.; Ramirez, J.; Sachleben, J.; Isikbay, M.; Singhal, H.; Greene, G.; Vander Griend, D.; Ratain, M. METABOLISM OF MEGESTROL ACETATE IN VITRO AND THE ROLE OF OXIDATIVE METABOLITES. Abstract. See: Poster Session I (PI-001-120) Displayed 7:30 Am – 2:00 Pm Attended 7:30 Am – 9:00 Am. Clin. Pharmacol. Ther. 2014, 95 (1), S17–S56. (View all citations for this reference)
- Toxnet: Megestrol Acetate (View all citations for this reference)
Excretion
Major route of elimination is through urine, minor route through feces.
Cmax
25-100 ng/mL from 50 mg oral dose
Tmax
2-5 h from 50 mg oral dose
Indications
Tablets: Palliative treatment of advanced carcinoma of the breast or endometrium. Should not be used in lieu of surgery, radiation, or chemotherapy, or other accepted procedures.
Oral suspension: anorexia, cachexia, or unexplained, significant weight loss in patients with AIDS.
Metabolites
Name
Structure
Notes
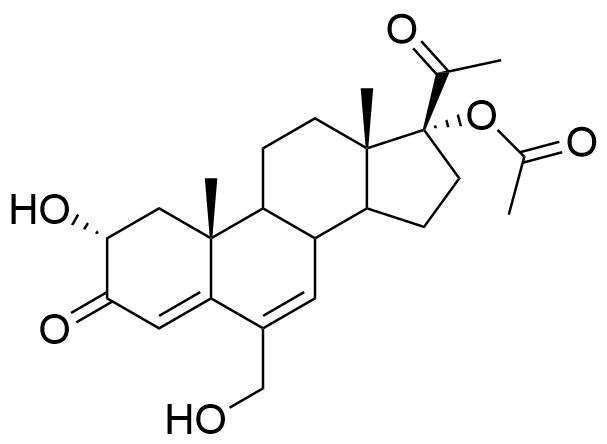
Metabolized in liver. Also found as glucuronide conjugate.
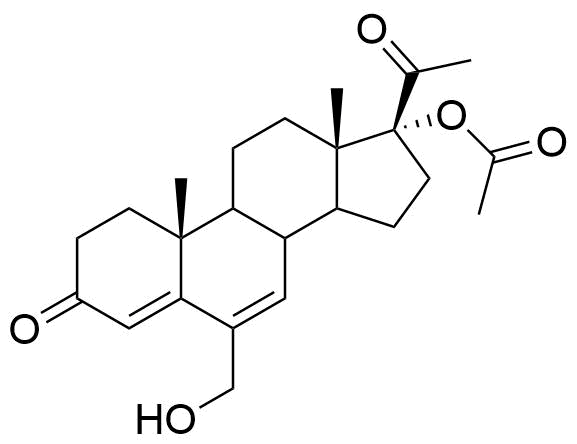
Metabolized in liver. Also found as glucuronide conjugate.
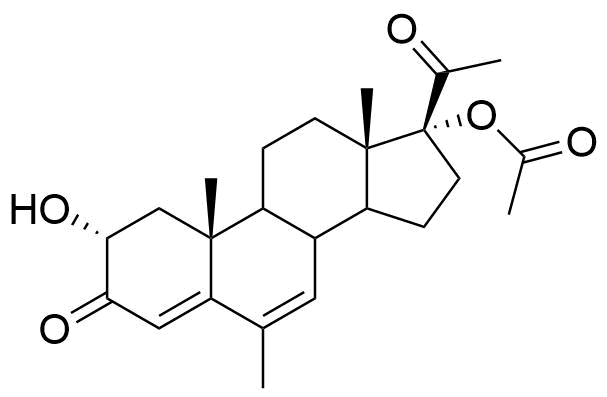
Metabolized in liver. Also found as glucuronide conjugate.
Two oxidative metabolites observed: MS-1 and MS-2. MS-1 identified as an alcohol, MS-2 is unknown. Their chemical structures were not specified.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
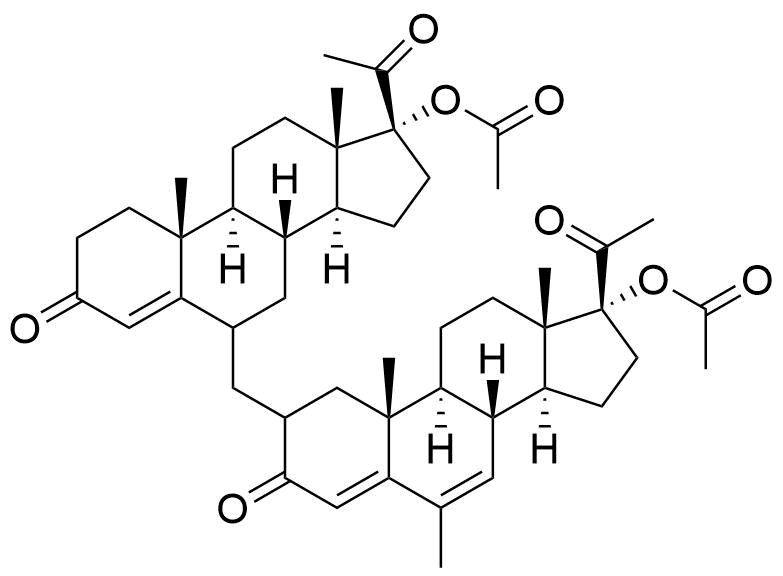
- BP Megestrol Acetate Impurity L
- 2ξ-[[17-(acetyloxy)-3,20-dioxopregn-4-en-6ξ-yl]methyl]-6-methyl-3,20-dioxopregna-4,6-dien-17-yl acetate