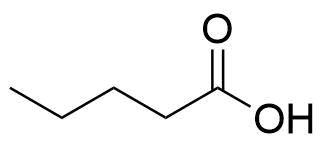
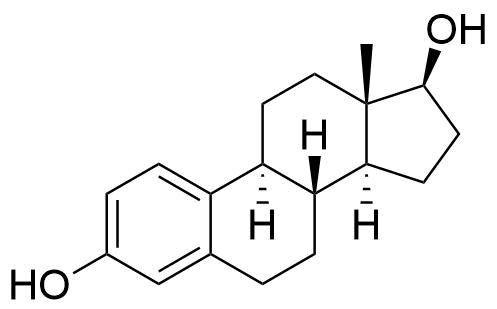
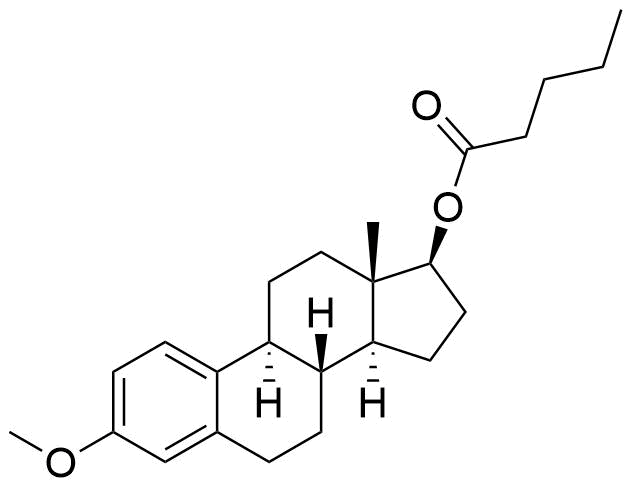
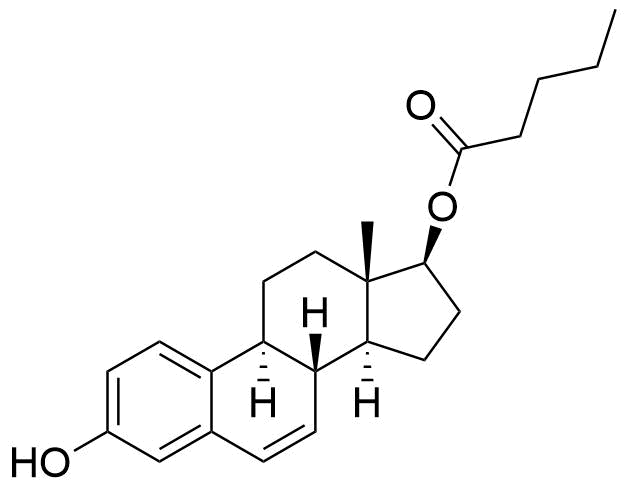
Estradiol Valerate
Estradiol Valerate (EV, E2V) is an ester prodrug of estradiol and is used in place of estradiol in some medications.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Estradiol Estradiol Cypionate
Identifiers
Abbreviation
EV, E2V
References
Names
- estra-1,3,5(10)-triene-3-17-diol(17β)-, 17-pentanoate
- estradiol 17β-valerate
- oestradiol valerate
References
CASRN
979-32-8
References
PubChem CID
13791
ECHA InfoCard
- 100.012.327
- EC / List #: 213-559-2
UNII
OKG364O896
KEGG Entry Number
D01413
Wikipedia Entry Name
Estradiol Valerate
ChEBI ID
CHEBI:31561
ChEMBL ID
CHEMBL1511
ChemSpider ID
13194
NIST
Estradiol Valerate
Physical & Chemical Properties
Molecular Formula
C23H32O3
References
Molecular Weight
356.50 g/mol
References
Melting Point
143°–150°
References
- USP 40: Estradiol Valerate monograph. (View all citations for this reference)
- Toxnet: Estradiol. (View all citations for this reference)
Specific Optical Rotation
+41° to +47°, 25 mg (uncorrected for moisture) per mL in dioxane
References
- USP 40: Estradiol Valerate monograph. (View all citations for this reference)
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360FD | May damage fertility. May damage the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
Toxicology
See estradiol.
References
MRTD
0.5 mg/kg/day
Biochemistry & Pharmacology
Pharmacology
See estradiol
References
Estrogen Receptor Activity
Agonist
Bioavailability
IV and intramuscular administration: 100%
Oral: 3%
References
- Dusterberg, B.; Nishino, Y., PHARMACOKINETIC AND PHARMACOLOGICAL FEATURES OF ESTRADIOL VALERATE. Maturitas 1982, 4 (4), 315-324. (View all citations for this reference)
Metabolism
Metabolized by CYP3A4, CYP2C9, UGT
Enzyme Interactions
Inhibits CYP2C19, CYP3A4, CYP2B6
Induces UGTs
Indications
Primary ovarian insufficiency, menorrhagia, hypogonadism, infertility
Metabolites
Impurities
Name
Structure
CASRN
Other Names & Identifiers
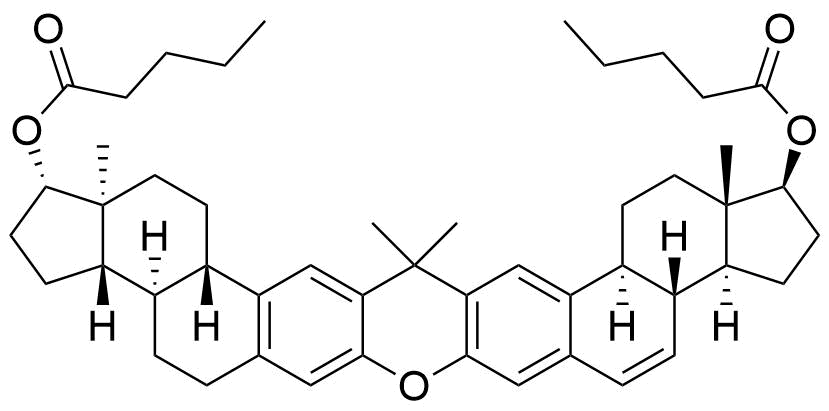
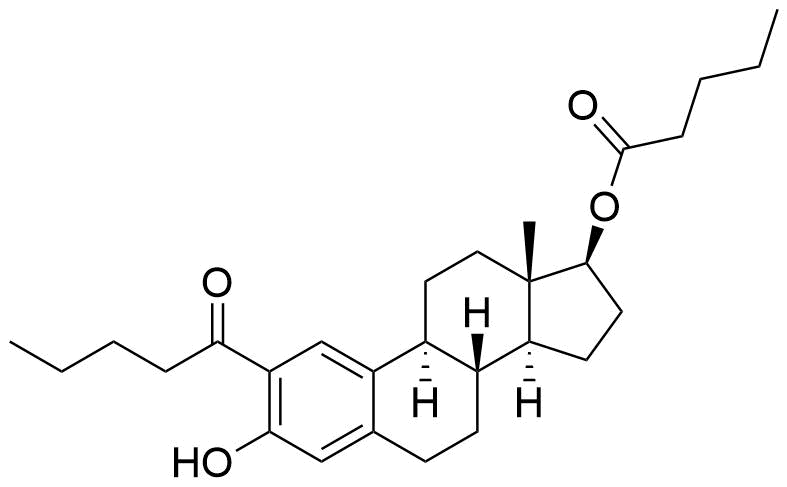
- BP Estradiol Valerate Impurity I
- (1S,3aS,3bR,10aR,10bS,13S,13aS,15aS,18bS,20aS)-13a,17,17,20a-tetramethyl-2,3,3a,3b,4,5,9,10,10a,10b,11,12,13,13a,14,15,15a,17,18b,19,20,20a-docosahydro-1H-bis(cyclopenta[5,6]naptho)[1,2-b:2',1'-i]xanthene-1,13-diyl dipentanoate
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Discontinued
086865
Discontinued
085860