Lynestrenol
Lynestrenol is a synthetic progestogen used in oral contraceptives and treatments of other disorders. It is a prodrug of norethindrone, therefore its pharmacology is almost identical to NET.
Tags
Approvals
WHO PrequalificationRelated Compounds
Norethindrone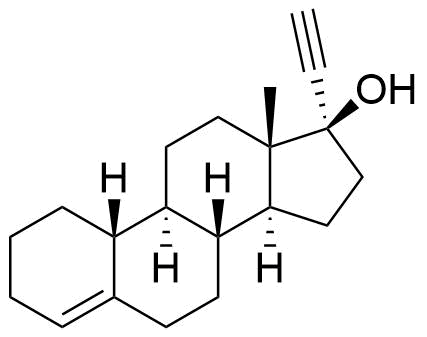
Identifiers
Names
- Linestrenol
- Lynenol
- 19-nor-17α-pregn-4-en-20-yn-17-ol
CASRN
57-76-6
References
ECHA InfoCard
- 100.000.139
- EC / List #: 200-151-4
UNII
N2Z8ALG4U5
KEGG Entry Number
D01580
Wikipedia Entry Name
Lynestrenol
ChemSpider ID
5648
ATC Code(s)
Physical & Chemical Properties
Molecular Formula
C20H28O
References
Molecular Weight
284.436 g/mol
References
Appearance
White, crystalline powder
Melting Point
158-160 °C
References
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Lynestrenol. (View all citations for this reference)
Solubility
Practically insoluble in water; soluble in ethanol, acetone and diethyl ether; freely soluble in chloroform
Specific Optical Rotation
-13 ° in chloroform
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
Genotoxicity
Possibly genotoxic and cytotoxic to mouse bone marrow cells.
Biochemistry & Pharmacology
Pharmacology
As a prodrug of norethindrone that forms no other metabolites, the pharmacology of lynestrenol is essentially the same as norethindrone.
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
Progesterone Receptor Activity
Agonist
Target Pathways
Serum Protein Binding
97% bound to plasma proteins
Metabolism
Metabolized by CYP2C9, CYP2C19, CYP3A4 into ethynodiol, then norethindrone.
References
- Korhonen, T.; Turpeinen, M.; Tolonen, A.; Laine, K.; Pelkonen, O. Identification of the Human Cytochrome P450 Enzymes Involved in the in Vitro Biotransformation of Lynestrenol and Norethindrone. J. Steroid Biochem. Mol. Biol. 2008, 110 (1–2), 56–66. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Enzyme Interactions
Inhibits CYP2C9
Bioequivalence
1 mg lynestrenol is bioequivalent to 0.7 mg norethindrone.
References
- Toxnet: Lynestrenol. (View all citations for this reference)
- Kuhl, H.; Bremser, H.-J.; Taubert, H.-D. Serum Levels and Pharmacokinetics of Norethisterone after Ingestion of Lynestrenol: Its Relation to Dose and Stage of the Menstrual Cycle. Contraception 1982, 26 (3), 303–315. (View all citations for this reference)
Metabolites
Name
Structure
Notes
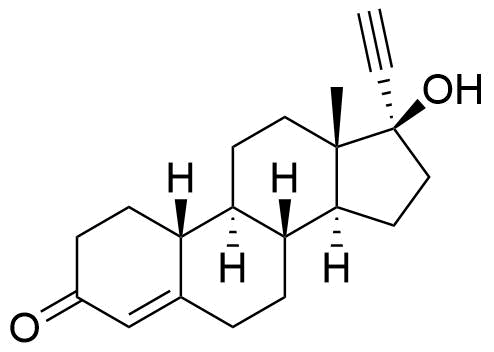
Norethindrone acetate, norethindrone enanthate, lynestrenol, and ethynodiol diacetate (and to a very small extent, norethynodrel) are prodrugs of norethindrone. Conversion of lynestrenol to ethynodiol to norethindrone facilitated by CYP2C9, CYP2C19, and CYP3A4. Conversion of norethynodrel to norethindrone is accomplished by either non-enzymatic or enzymatic ketosteroid isomerization.