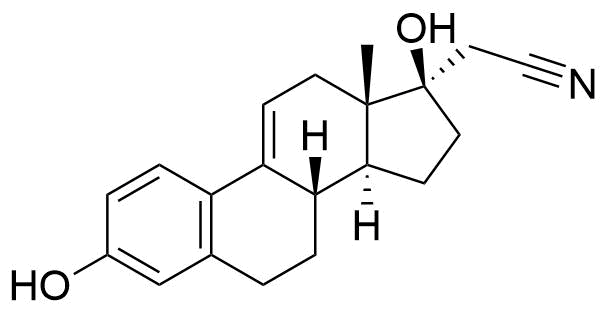
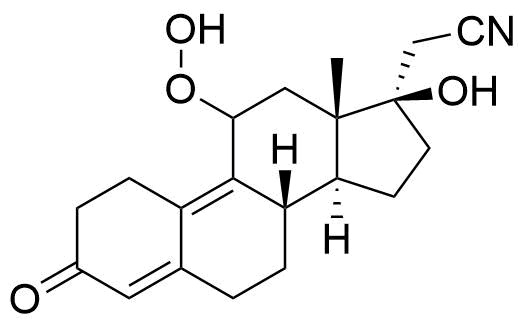
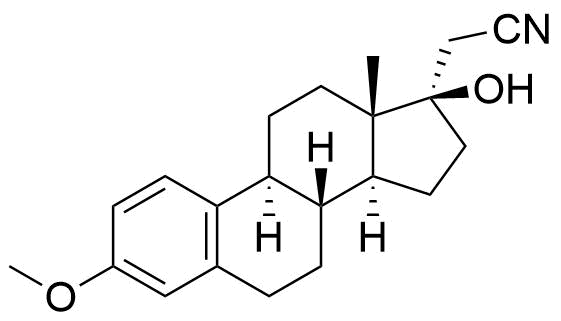
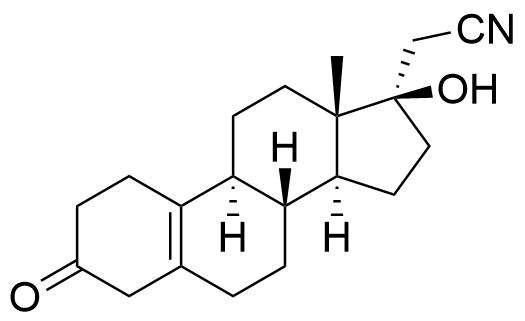
Dienogest
Dienogest (DNG) is a progestogen used in hormonal contraceptives and for the treatment of endometriosis.
Tags
Approvals
US FDA-Approved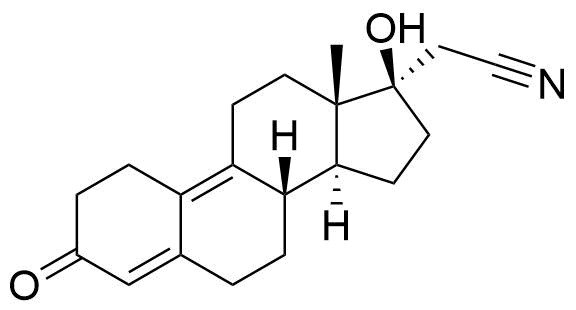
Identifiers
Abbreviation
DNG
References
Names
- M-18575
- MJR-35
- SH-660
- STS-557
- ZK-37659
- 17α-cyanomethyl-17β-hydroxy-estra-4,9(10)-dien-3-one
- 17-hydroxy-3-oxo-19-nor-17α-pregna-4,9-diene-21-nitrile
- (17-hydroxy-3-oxoestra-4,9-dien-17α-yl)acetonitrile
- Cyanomethyldienolone
CASRN
65928-58-7
References
PubChem CID
68861
ECHA InfoCard
- 100.167.087
- EC /List #: 639-448-2
IUPHAR/BPS
7654
DrugBank Accession Number
DB09123
References
- DrugBank: Dienogest
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
46M3EV8HHE
KEGG Entry Number
D03799
Wikipedia Entry Name
Dienogest
ChEBI ID
CHEBI:70708
ChEMBL ID
CHEMBL1201864
ChemSpider ID
62093
ATC Code(s)
Physical & Chemical Properties
Molecular Formula
C20H25NO2
References
Molecular Weight
311.43 g/mol
References
Appearance
White, almost white, or slightly yellow crystalline powder
References
- BP 2017: Dienogest monograph. (View all citations for this reference)
Melting Point
144-145
Solubility
Practically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in methanol
References
- BP 2017: Dienogest monograph. (View all citations for this reference)
Specific Optical Rotation
-352 to -344, 0.125 g dried substance in methanol, diluted to 25.0 mL
References
- BP 2017: Dienogest monograph. (View all citations for this reference)
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360 | May damage fertility or the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children |
Side Effects
In COCs with ethinyl estradiol: irregular vaginal bleeding, headache, breast pain, nausea/vomiting, depression, decreased libido
References
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Carcinogenicity
No evidence in vivo and in vitro.
References
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
Mutagenicity
No evidence in vivo and in vitro.
References
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
LD50
- mouse oral: 4 g/kg
- rabbit oral: 1600 mg/kg
- LDLo mouse intraperitoneal: 1 g/kg
- LDLo mouse subcutaneous: 5 g/kg
- LDLo rabbit intraperitoneal: 1.5 g/kg
- LDLo rabbit oral: 1 g/kg
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist. Some antagonist behavior.
References
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Androgen Receptor Activity
Antagonist (40% of CPA, the most potent anti-androgenic progestin)
References
- Sitruk-Ware, R.; El-Etr, M., Progesterone and related progestins: potential new health benefits. Climacteric 2013, 16, 69-78. (View all citations for this reference)
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Sitruk-Ware, R.; Small, M.; Kumar, N.; Tsong, Y.-Y.; Sundaram, K.; Jackanicz, T., Nestorone®: clinical applications for contraception and HRT. Steroids 2003, 68 (10-13), 907-913. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Bartsch, V., Gynaecological uses of dienogest alone and in combination with oestrogens. Journal of Medical Drug Reviews 2015, 5, 1-31. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Sitruk-Ware, R., New progestagens for contraceptive use. Hum. Reprod. Update 2006, 12 (2), 169-78. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Estrogen Receptor Activity
Weak or no agonist and antagonist activity
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Sitruk-Ware, R., New progestagens for contraceptive use. Hum. Reprod. Update 2006, 12 (2), 169-78. (View all citations for this reference)
- Bartsch, V., Gynaecological uses of dienogest alone and in combination with oestrogens. Journal of Medical Drug Reviews 2015, 5, 1-31. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Glucocorticoid Receptor Activity
Low to no activity
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Mineralocorticoid Receptor Activity
No activity
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Target Pathways
Bioavailability
90-96.2%
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Bartsch, V., Gynaecological uses of dienogest alone and in combination with oestrogens. Journal of Medical Drug Reviews 2015, 5, 1-31. (View all citations for this reference)
- Stanczyk, F. Z., Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev. Endocr. Metab. Disord. 2002, 3 (3), 211-224. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Elimination Half-Life (t1/2)
8-10 h
References
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Stanczyk, F. Z., Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev. Endocr. Metab. Disord. 2002, 3 (3), 211-224. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Serum Protein Binding
90% bound to serum albumin, 10% free. No binding to SHBG or CBG.
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Bartsch, V., Gynaecological uses of dienogest alone and in combination with oestrogens. Journal of Medical Drug Reviews 2015, 5, 1-31. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Metabolism
CYP3A4
Excretion
Mainly via kidneys.
References
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Apparent Volume of Distribution
40 L from 2 mg/day oral dose
References
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Tmax
2 h
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Clearance
Oettel et al: 75 mL/h/kg
Foster et al: 3 L/h from 2 mg/day oral dose
References
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
Enzyme Interactions
3β-hydroxysteroid dehydrogenase-Δ5-4-isomerase: inhibitor
References
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
Minimum Contraceptive Threshold
Oettel: 4.0 ng/mL
Foster: 1 mg/day oral dose for inhibition of ovulation in cyclical women
References
- Oettel, M.; Breitbarth, H.; Elger, W.; Gräser, T.; Hübler, D.; Kaufmann, G.; Moore, C.; Patchev, V.; Römer, W.; Schröder, J.; et al. The Pharmacological Profile of Dienogest. Eur. J. Contracept. Reprod. Heal. Care 1999, 4 (sup1), 2–13. (View all citations for this reference)
- Foster, R. H.; Wilde, M. I. Dienogest. Drugs 1998, 56 (5), 825–833. (View all citations for this reference)
Indications
Indicated for use by women for pregnancy prevention. Also indicated for the treatment of heavy menstrual bleeding in women without organic pathology who choose to use an oral contraceptive as their method of birth control. (PubChem) Menorrhagia, endometriosis (ChEMBL)
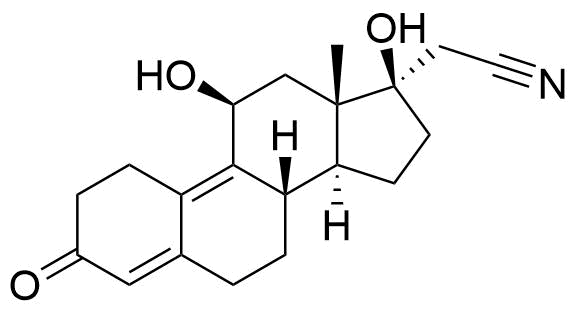
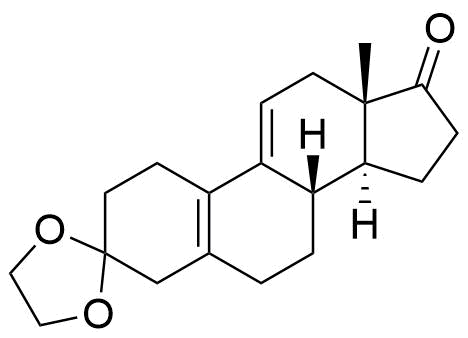
Metabolites
Name
Structure
Notes
A number of other metabolites have been detected, including unspecified hydrogenation and hydroxylation products.
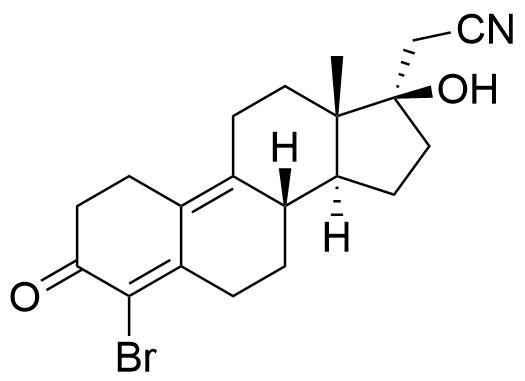
Impurities
Name
Structure
CASRN
Other Names & Identifiers
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Prescription
022252
None (Tentative Approval)
202999
Prescription
202349