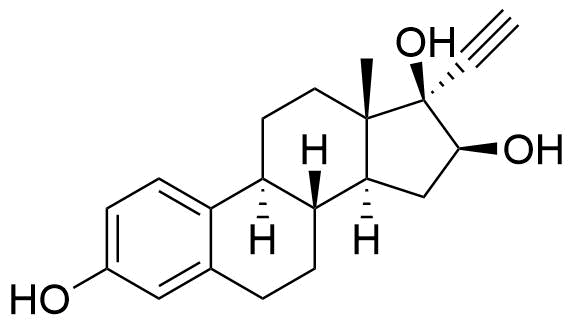
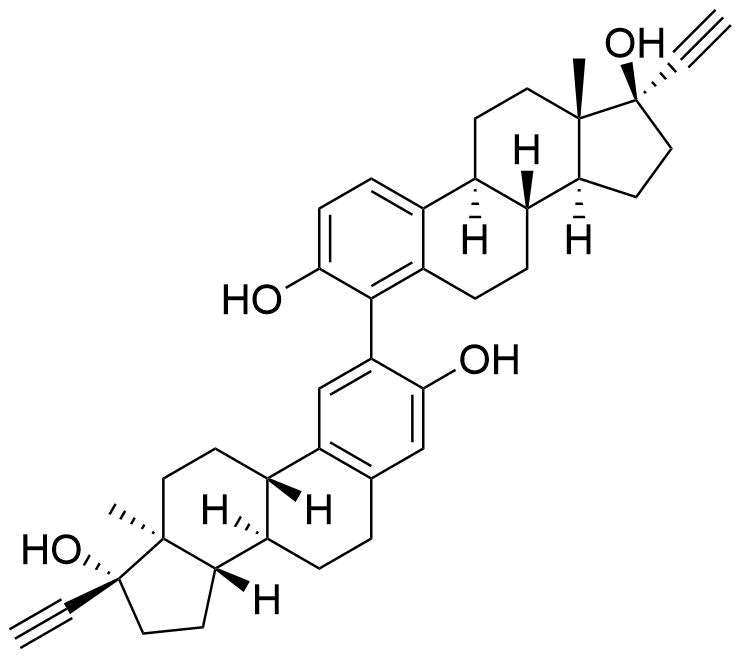
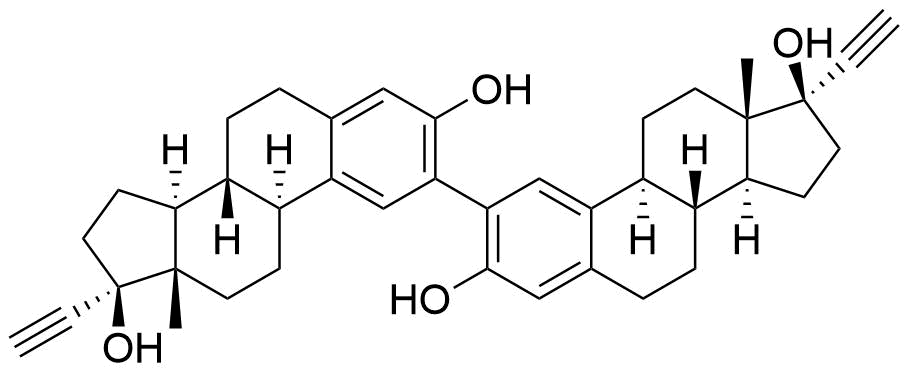
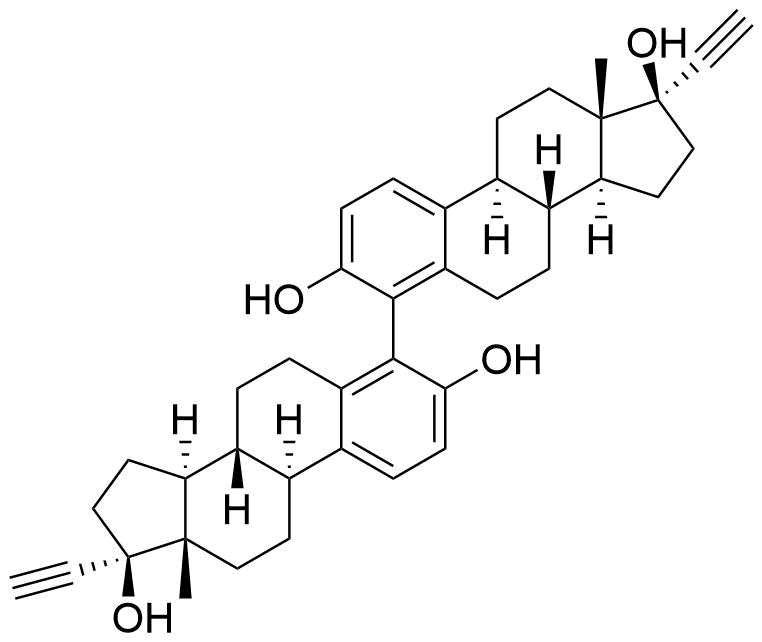
Ethinyl Estradiol
Ethinyl estradiol (EE) is a synthetic estrogen derivative used alongside progestins in a large majority of combined oral contraceptives for better cycle control and to help prevent ovulation.
Tags
Approvals
WHO Essential Medicine WHO Prequalification US FDA-Approved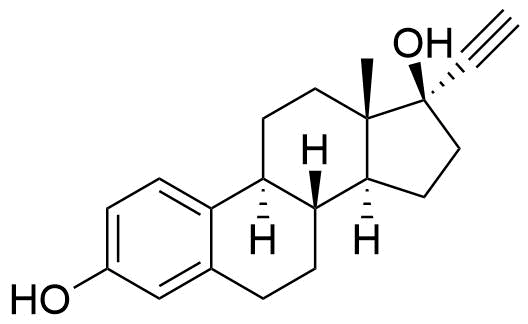
Identifiers
Abbreviation
EE
References
Names
- Ethinylestradiol
- 17α-ethynylestradiol
- 17α-ethynylestra-1,3,5(10)-triene-3,17β-diol
- NSC-10973
- 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol
- aethinyloestradiol
References
CASRN
57-63-6
References
PubChem CID
5991
ECHA InfoCard
- 100.000.311
- EC / List #: 200-32-2
IUPHAR/BPS
7071
DrugBank Accession Number
DB00977
References
- DrugBank: Ethinyl Estradiol.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
423D2T571U
KEGG Entry Number
D00554
Wikipedia Entry Name
Ethinylestradiol
ChEBI ID
CHEBI:4903
ChemSpider ID
5770
PDB
Ethinyl Estradiol
NIST
Ethinyl Estradiol
ATC Code(s)
Physical & Chemical Properties
Molecular Formula
C20H24O2
References
Molecular Weight
296.403 g/mol
References
Appearance
White or slightly yellowish-white, crystalline powder
References
- British Pharmacopoeia 2017: Ethinylestradiol monograph (View all citations for this reference)
Melting Point
180-186 °C (if polymorphic modification: 142-146 °C)
References
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- Ethinyl Estradiol. Toxicology Data Network. US National Library of Medicine [Online Database] (View all citations for this reference)
- USP 40: Ethinyl Estradiol monograph. (View all citations for this reference)
Solubility
BP: practically insoluble in water.
PubChem: 11.3 mg/L water at 27 °C.
PubChem, BP:
1 part in 6 of ethanol, 1 in 4 of ether, 1 in 5 of acetone, 1 in 4 of dioxane, and 1 in 20 of chloroform. Soluble in vegetable oils, and in solutions of fixed alkali hydroxides (e.g. NaOH, KOH)
References
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- Ethinyl Estradiol. Toxicology Data Network. US National Library of Medicine [Online Database] (View all citations for this reference)
- ChemIDPlus: A Toxnet Database. Ethinylestradiol. (View all citations for this reference)
- British Pharmacopoeia 2017: Ethinylestradiol monograph (View all citations for this reference)
logP
3.67
Specific Optical Rotation
Pubchem/Toxnet:
[3.5°] at 24° C/D (c = 2 in dioxane); [-29.5°] at 24° C/D (c = 2 in pyridine)
USP: -28° to -29.5°, 50 mg/mL in colorless pyridine from a freshly opened container
References
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- Ethinyl Estradiol. Toxicology Data Network. US National Library of Medicine [Online Database] (View all citations for this reference)
- USP 40: Ethinyl Estradiol monograph. (View all citations for this reference)
Vapor Pressure
1.95x10-9 mm Hg at 25° C (est)
Henry's Law Constant
7.94x10-12 atm-cu m/mol at 25° C (est.)
Polymorphism
Yes
References
- British Pharmacopoeia 2017: Ethinylestradiol monograph (View all citations for this reference)
Storage Conditions
Protect from light
References
- British Pharmacopoeia 2017: Ethinylestradiol monograph (View all citations for this reference)
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Carcinogenicity | 1B | H350 | May cause cancer |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360 | May damage fertility or the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Specific Target Organ Toxicity, Repeated Exposure | 1 | H372 | Causes damage to organs through prolonged or repeated exposure |
Side Effects
Adverse effects attributed to estrogenic and metabolic effects. Water and sodium retention, which may lead to weight gain, edema, and tender breast enlargement. Changes in libido and withdrawal vaginal bleeding. Liver function impairment, jaundice, and gallstones may occur. Headache, depression, dizziness, glucose intolerance, and a sensitivity to contact lenses have been reported. Nausea, vomiting and break through vaginal bleeding are not uncommon. Dermatological effects include chloasma, melasma, rashes, and urticaria. Erytheme multiforme and erytheme nodosum occur. Hypertension and thromoboembolic disease are reported.
Ortho Evra adverse effects: breast symptoms, headache, application site reaction, nausea, upper respiratory infection, menstrual cramps, abdominal pain.
References
- Ethinyl Estradiol. Toxicology Data Network. US National Library of Medicine [Online Database] (View all citations for this reference)
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- FDA Ortho Evra Information (View all citations for this reference)
Carcinogenicity
There is sufficient evidence in humans for the carcinogenicity of post-menopausal estrogen therapy.
Mutagenicity
Not found to be mutagenic in the Ames Salmonella/microsome direct plate incorporation protocol.
References
- Lang, R.; Reimann, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. I. Communication: Examination for the Induction of Gene Mutations Using the Ames Salmonella/microsome Test and the HGPRT Test in V79 Cells. Environ. Mol. Mutagen. 1993, 21 (3), 272–304. (View all citations for this reference)
Genotoxicity
Not found to be genotoxic in human lymphocyte assay in vitro and mouse bone marrow micronucleus test in vivo.
References
- (1) Reimann, R.; Kalweit, S.; Lang, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. II. Communication: Examination for the Induction of Cytogenetic Damage Using the Chromosomal Aberration Assay on Human Lymphocytes in Vitro and the Mouse Bone Marrow Micronucleus Test in vivo. Environ. Mol. Mutagen. 1996, 28 (2), 133–144. (View all citations for this reference)
LD50
- mouse intraperitoneal: 0.250 g/kg
- mouse oral: 0.950 g/kg (ChemIDPlus), 1737 mg/kg (PubChem)
- mouse subcutaneous: > 3 g/kg
- rat intraperitoneal: 0.471 g/kg
- rat oral: 0.960 g/kg (PubChem: 1200 mg/kg and > 5000 mg/kg)
- rat subcutaneous: > 2 g/kg
TDLo
human women: 0.21 mg/kg/21 days
TD50
rat, hepatic tumor: 0.2 mg/kg/day
MRTD
0.0005 mg/kg/day
Biochemistry & Pharmacology
Estrogen Receptor Activity
Agonist
References
- Jie Shen, Lei Xu, Hong Fang, Ann M Richard, Jeffrey D Bray, Richard S Judson, Guangxu Zhou,Thomas J Colatsky, Jason L Aungst, Christina Teng, Stephen C Harris, Weigong Ge, Susie Y Dai, Zhenqiang Su, Abigail C Jacobs, Wafa Harrouk, Roger Perkins, Weida Tong, Huixiao Hong. "EADB: An Estrogenic Activity Database for Assessing Potential Endocrine Activity," Toxicological Science 2013, 135(2), 277-291 (View all citations for this reference)
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
Bioavailability
- Oral: Around 45%
- Vaginal Ring: 55%
References
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Stanczyk FZ. All progestins are not created equal. Steroids. 2003; 68:879-90. (View all citations for this reference)
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- Back, D.; Madden, S.; Orme, M. Gastrointestinal Metabolism of Contraceptive Steroids. Int. J. Gynecol. Obstet. 1991, 36 (3), 260–260. (View all citations for this reference)
Elimination Half-Life (t1/2)
- PubChem: 13-27 h (35 μg ethinyl estradiol with 1 mg norethindrone)
- 7.7 h (single oral dose)
- PubChem: 36 +/- 13 h
References
- Ethinyl Estradiol. Toxicology Data Network. US National Library of Medicine [Online Database] (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
Serum Protein Binding
97% bound. Mainly to serum albumin. No binding to SHBG, but induces SHBG synthesis.
References
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- Ethinyl Estradiol. Toxicology Data Network. US National Library of Medicine [Online Database] (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- FDA Ortho Evra Information (View all citations for this reference)
Metabolism
Metabolized by CYP3A4, CYP2C9, UGT
Excretion
Primarily in urine with sulfate and glucuronide conjugates. Some fecal excretion. Very small amounts excreted in milk.
Tmax
2-3 h
Enzyme Interactions
- Inhibits CYP2C9, CYP2C19, CYP3A4, CYP2B6
- After formation of very reactive intermediate by oxidation of C17 ethinyl, can irreversibly inhibit cytochrome P450 enzymes (e.g. CYP2C8, CYP3A4). Thus it exerts strong effects on hepatic metabolism.
- Nuclear receptor subfamily 1 group I member 2: agonist
- Bile salt export pump: inhibitor, inducer
- Sodium/bile acid cotransporter: inducer
- Canalicular multispecific organic anion transporter 1: inducer
- Multidrug resistance protein 1: substrate
- Uridine diphosphate glucuronosyltransferase (UGT): inducer
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- DrugBank: Ethinyl Estradiol.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - KEGG: Ethinyl Estradiol (View all citations for this reference)
- National Center for Biotechnology Information. PubChem Compound Database; CID=5991. (View all citations for this reference)
- Laine, K.; Yasar, U.; Widen, J.; Tybring, G. A Screening Study on the Liability of Eight Different Female Sex Steroids to Inhibit CYP2C9, 2C19 and 3A4 Activities in Human Liver Microsomes. Pharmacol. Toxicol. 2003, 93 (2), 77–81. (View all citations for this reference)
- Nanda, K.; Stuart, G. S.; Robinson, J.; Gray, A. L.; Tepper, N. K.; Gaffield, M. E. Drug Interactions between Hormonal Contraceptives and Antiretrovirals. AIDS 2017, 31 (7), 917–952. (View all citations for this reference)
Indications
For treatment of moderate to severe vasomotor symptoms associated with menopause, female hypogonadism, prostatic carcinoma-palliative therapy of advanced disease, breast cancer, as an oral contraceptive, and as emergency contraceptive.
Metabolites
Name
Structure
Notes
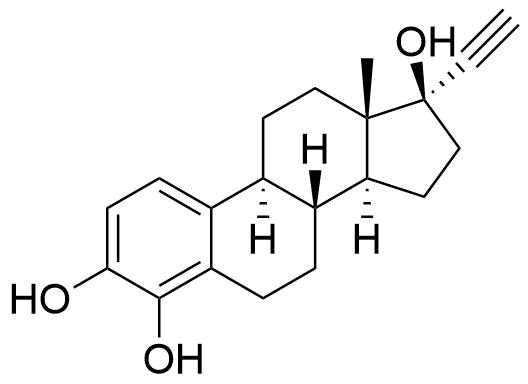
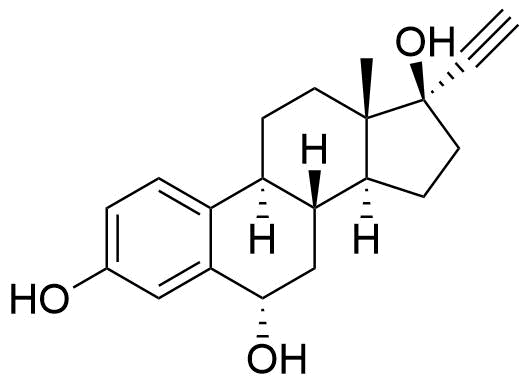
Contributes to metabolism only to a small extent. Can be converted to methoxy EE by catechol O-methyltransferase.
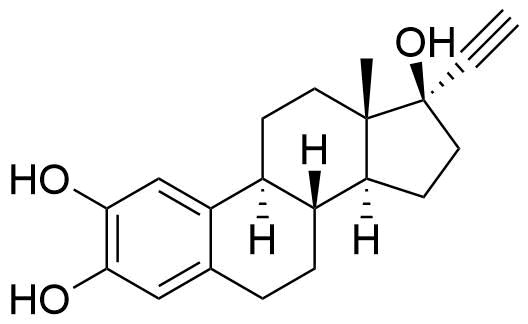
Major metabolite. Formed by CYP3A4. Can be converted to the methoxy EE by catechol O-methyltransferase.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
US FDA-Approved Products
Name
Formulation
Status
ANDA #
WHO Essential Medicines
Name
Formulation
LEVONORGESTREL: 1 mg
Tablet
LEVONORGESTREL: 150 μg
Tablet
WHO Prequalified Medicines
WHO Reference #
Name
Applicant
Formulation
ETHINYL ESTRADIOL: 30 μg
Tablet
Placebo tablet: 0 mg
ETHINYL ESTRADIOL: 30 μg
Tablet
Placebo tablet: 0 mg
LEVONORGESTREL: 0.15 mg
Tablet
Placebo tablet: 0 mg
LEVONORGESTREL: 0.15 mg
Tablet
Placebo Tablet: 0 mg
LEVONORGESTREL: 150 μg
Tablet
Placebo (Ferrous Fumarate Tablet): 75 mg