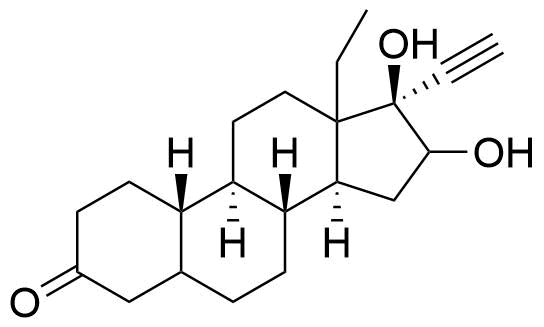
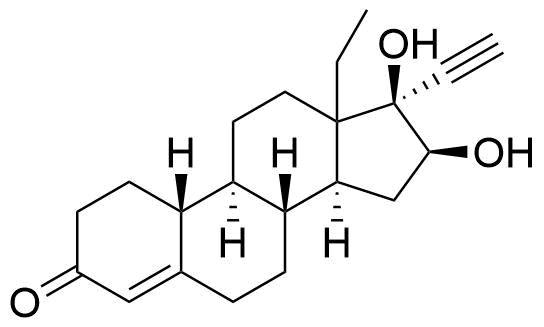
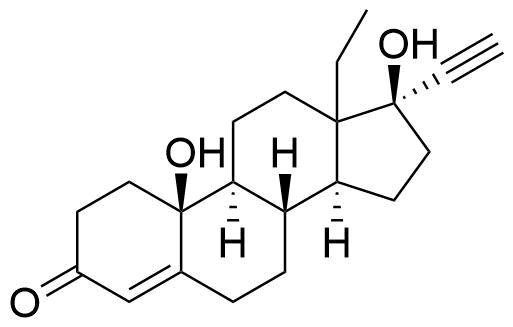
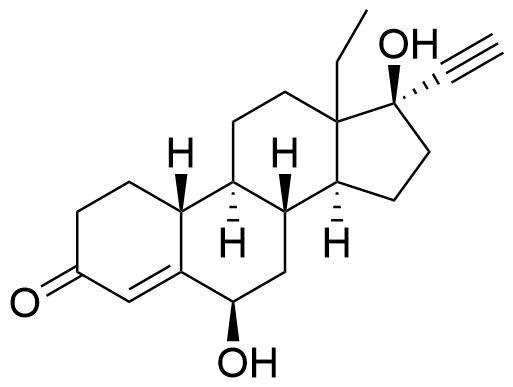
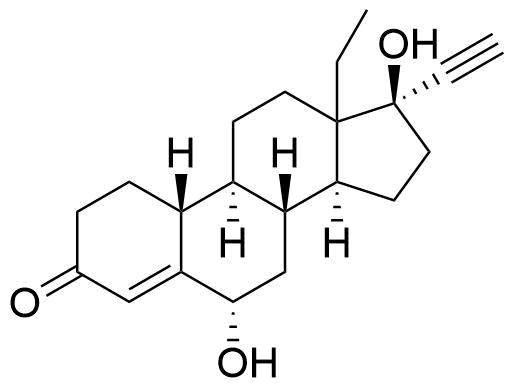
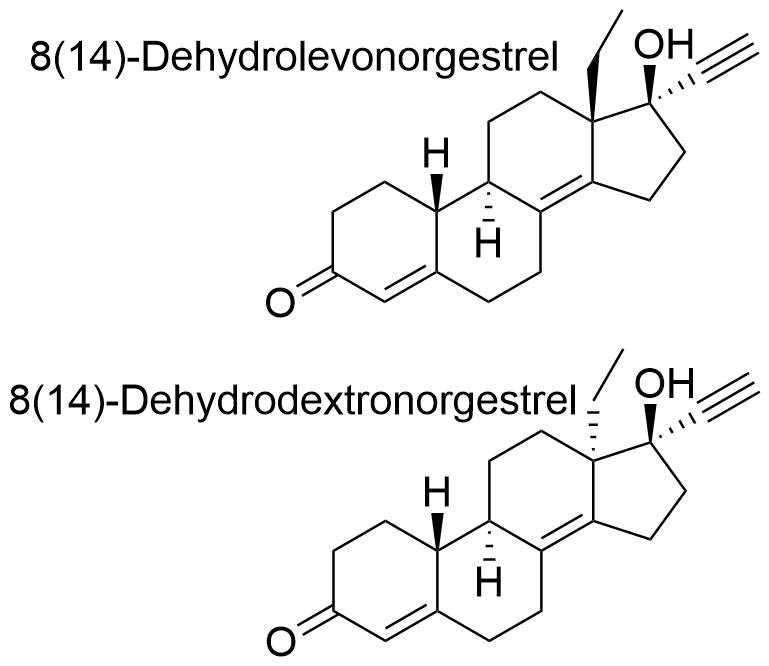
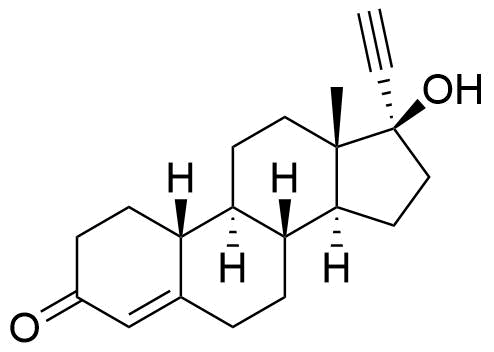
Norgestrel
Norgestrel is a synthetic progestogen used in combined oral contraceptives. It is a racemic mix of the stereoisomers dextronorgestrel and levonorgestrel. As dextronorgestrel is biologically inactive, the behavior of norgestrel is identical to that of levonorgestrel.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Levonorgestrel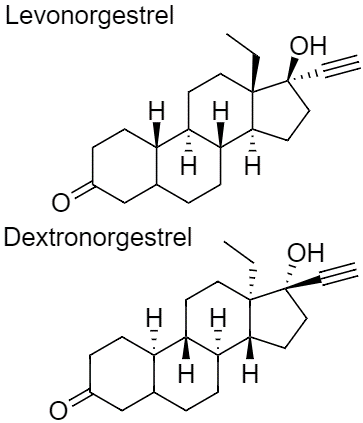
Identifiers
CASRN
6533-00-2
References
PubChem CID
16051930
ECHA InfoCard
- 100.026.758
- EC / List #: 229-433-5
DrugBank Accession Number
DB09389
References
- DrugBank: Norgestrel.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
3J8Q1747Z2
KEGG Entry Number
D00954
Wikipedia Entry Name
Norgestrel
ChEBI ID
CHEBI:7630
ChemSpider ID
10481953
ATC Code(s)
References
- DrugBank: Norgestrel.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C21H28O2
References
Molecular Weight
312.45 g/mol
References
Appearance
White or almost white crystalline powder
References
- BP 2017: Norgestrel monograph. (View all citations for this reference)
Solubility
Practically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in alcohol.
References
- BP 2017: Norgestrel monograph. (View all citations for this reference)
Specific Optical Rotation
BP: +0.05° to -0.05°. 0.5 g in methylene chloride diluted to 10.0 mL
IARC: -0.1 to +0.1° in chloroform
References
- BP 2017: Norgestrel monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360FD | May damage fertility. May damage the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children |
Genotoxicity
Found to affect genetic material of human lymphocyte chromosomes as measured by chromosomal aberrations, sister chromatid exchanges, and cell growth kinetics.
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist
Target Pathways
Serum Protein Binding
Binds to SHBG
References
- Abrams, L. S.; Skee, D.; Natarajan, J.; Wong, F. A. Pharmacokinetic Overview of Ortho Evra (TM)/Evra (TM). Fertil. Steril. 2002, 77 (2, 2), S3–S12. (View all citations for this reference)
Metabolism
CYP3A4
Clearance
458 L/day
Metabolites
Impurities
Name
Structure
CASRN
Other Names & Identifiers
US FDA-Approved Products
Name
Formulation
Status
ANDA #
NORGESTREL: 0.5 mg
28 oral tablets
Prescription
075406
NORGESTREL: 0.5 mg
21 oral tablets
Discontinued
075406
Discontinued
202875