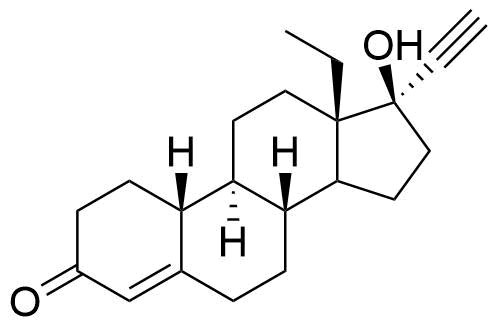
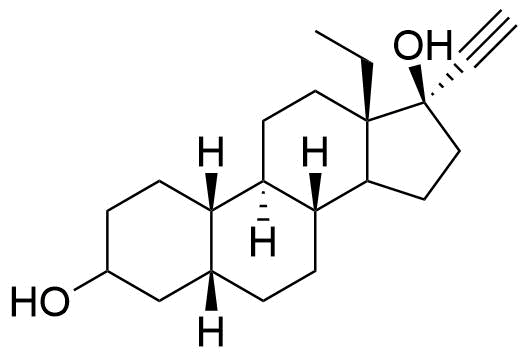
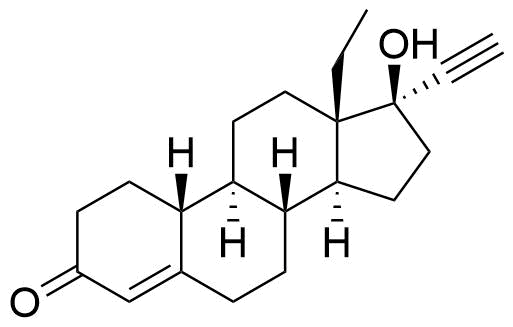
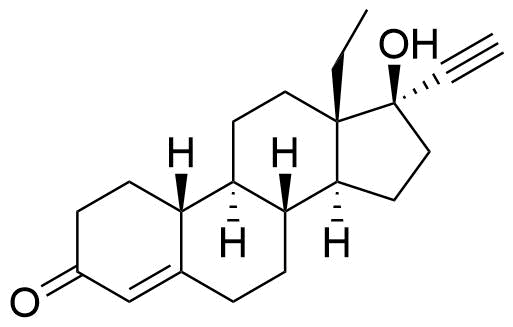
Norgestimate
Norgestimate is a synthetic progestogen used in combined oral contraceptives and hormone replacement therapy. It is a prodrug of norelgestromin, and, to a much lesser extent, levonorgestrel.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Norelgestromin Levonorgestrel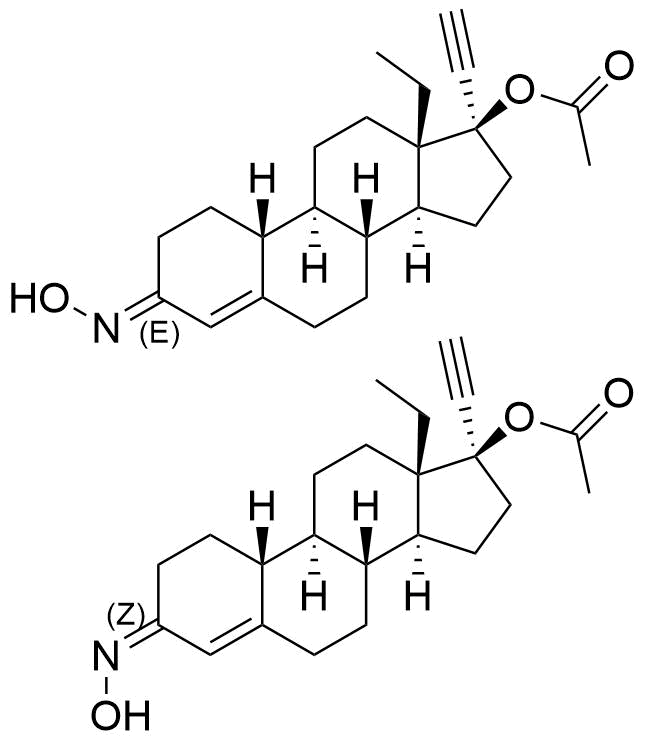
Identifiers
Abbreviation
NGM
References
- Wong, F. A.; Edom, R. W.; Duda, M.; Tischio, J. P.; Huang, M.; Juzwin, S.; Tegegne, G. Determination of Norgestimate and Its Metabolites in Human Serum Using High-Performance Liquid Chromatography with Tandem Mass Spectrometric Detection. J. Chromatogr. B Biomed. Sci. Appl. 1999, 734 (2), 247–256. (View all citations for this reference)
Names
- (3EZ)-13β-ethyl-3-(hydroxyimino)-18,19-dinor-17α-pregn-4-en-20-yn-17-yl acetate
- ORF-10131
- Norgestrel acetate oxime
References
CASRN
35189-28-7
References
PubChem CID
6540478
IUPHAR/BPS
7091
DrugBank Accession Number
DB00957
References
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
C291HFX4DY
KEGG Entry Number
D05209
Wikipedia Entry Name
Norgestimate
ChEBI ID
CHEBI:50815
ChEMBL ID
CHEMBL1200934
ATC Code(s)
References
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C23H31NO3
References
Molecular Weight
369.497 g/mol
References
Appearance
White or almost white powder
References
- British Pharmacopoeia 2017: Norgestimate monograph (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Melting Point
216° C
IARC, Toxnet: 214-218 °C
References
- ChemIDPlus: A Toxnet Database. Norgestimate. (View all citations for this reference)
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Norgestimate. (View all citations for this reference)
Solubility
Practically insoluble in water, freely soluble in methylene chloride, soluble in acetone, sparingly soluble in acetonitrile.
References
- British Pharmacopoeia 2017: Norgestimate monograph (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
logP
4.8
References
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Specific Optical Rotation
USP, IARC: +40° to +46°, 10 mg/mL in chloroform
BP: +42° to +50°, 0.200 g in 20.0 mL methylene chloride
Toxnet: +110 ° at 25 °C in unspecified solvent
References
- USP 40: Norgestimate monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Norgestimate. (View all citations for this reference)
Storage Conditions
Store at 25 °C, do no go outside of range 15-30 °C.
Ratio of (E)- to (Z)- Isomer
1.27 to 1.78
References
- British Pharmacopoeia 2017: Norgestimate monograph (View all citations for this reference)
Biochemistry & Pharmacology
Progesterone Receptor Activity
Partial agonist
References
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Paris, F.; Balaguer, P.; Rimbault, F.; Gaspari, L.; Sultan, C. Molecular Action of Norgestimate: New Developments. Gynecol. Endocrinol. 2015, 31 (6), 487–490. (View all citations for this reference)
Androgen Receptor Activity
Reports vary: "minimal androgenic activity" (Phillips), agonist (Ruan, Lello), partial agonist (DrugBank).
References
- Phillips A, Hahn DW, McGuire JL (1992). "Preclinical evaluation of norgestimate, a progestin with minimal androgenic activity". Am. J. Obstet. Gynecol. 167 (4 Pt 2): 1191–6. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
Estrogen Receptor Activity
Antagonist (Ruan, Lello). Selective agonist for ERα (Paris).
References
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Paris, F.; Balaguer, P.; Rimbault, F.; Gaspari, L.; Sultan, C. Molecular Action of Norgestimate: New Developments. Gynecol. Endocrinol. 2015, 31 (6), 487–490. (View all citations for this reference)
Glucocorticoid Receptor Activity
Pronounced as "unknown" by Stanczyk in 2013, but previously claimed as not active (Ruan, Lello). Recently (2015) defined as full antagonist with low affinity (Paris).
References
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Paris, F.; Balaguer, P.; Rimbault, F.; Gaspari, L.; Sultan, C. Molecular Action of Norgestimate: New Developments. Gynecol. Endocrinol. 2015, 31 (6), 487–490. (View all citations for this reference)
Mineralocorticoid Receptor Activity
Varies: Full antagonist with moderate activity (Paris). No activity (Ruan, Lello).
References
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Paris, F.; Balaguer, P.; Rimbault, F.; Gaspari, L.; Sultan, C. Molecular Action of Norgestimate: New Developments. Gynecol. Endocrinol. 2015, 31 (6), 487–490. (View all citations for this reference)
Target Pathways
Serum Protein Binding
Metabolites bind at >97%. Norelgestromin binds to albumin and not SHBG, while norgestrel binds primarily to SHBG.
Metabolism
Hepatic, CYP3A4
Excretion
Metabolites excreted via fecal and renal pathways.
Inhibition of Ovulation
0.25 mg/day
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Transformation of Endometrium
5-10 mg/cycle
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Menstrual Delay
5 mg/day
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Indications
Dysfunctional uterine bleeding, dysmenorrhea, hirsutism, hypermenorrhea, postmenopausal osteoporosis, moderate acne vulgaris, moderate & severe vulvovaginal atrophy, moderate & severe vasomotor symptoms
References
- DrugBank: Norgestimate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Metabolites
Name
Structure
Notes
Other metabolites include various hydroxylated and conjugated species.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
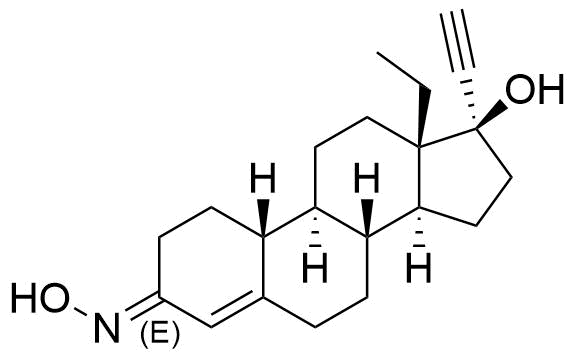
- BP Norgestimate Impurity C
- (3E)-13β-ethyl-3-(hydroxyimino)-18,19-dinor-17α-pregn-4-en-20-yn-17-ol
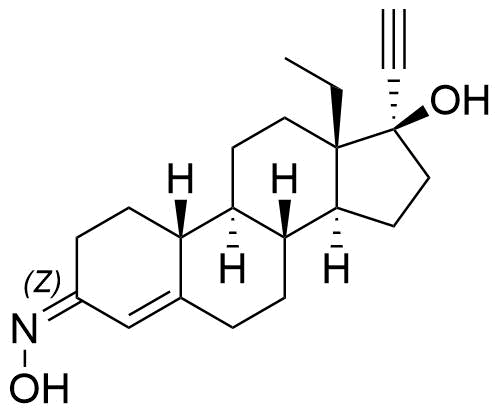
- BP Norgestimate Impurity D
- (3Z)-13β-ethyl-3-(hydroxyimino)-18,19-dinor-17α-pregn-4-en-20-yn-17-ol
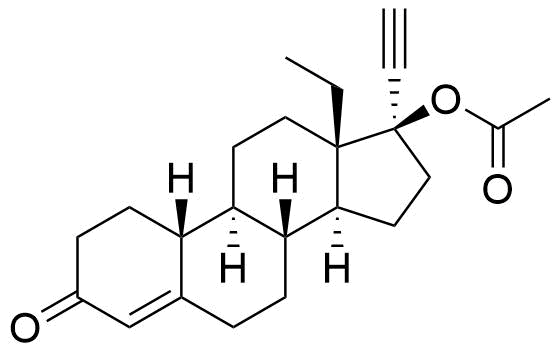
- BP Norgestimate Impurity A
- 13β-ethyl-3-oxo-18,19-dinor-17α-pregn-4-en-20-yn-17-yl acetate
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Prescription
076812
Prescription
075808
Prescription
076335
Prescription
205441