Levonorgestrel
Structure
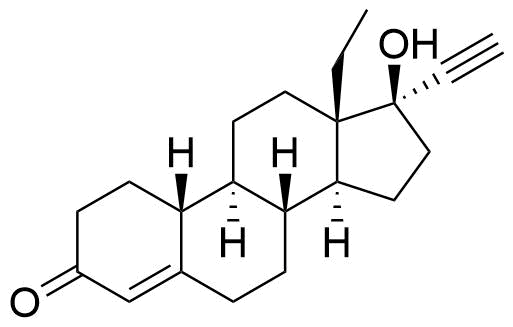
CASRN
797-63-7
Notes
An active metabolite of norelgestromin and a secondary metabolite of norgestimate.
References
- Thomas L. Lemke; David A. Williams; Victoria F. Roche; S. William Zito (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 1403. ISBN 978-1-60913-345-0. (View all citations for this reference)
- Hammond, G. L.; Abrams, L. S.; Creasy, G. W.; Natarajan, J.; Allen, J. G.; Siiteri, P. K. Serum Distribution of the Major Metabolites of Norgestimate in Relation to Its Pharmacological Properties. Contraception 2003, 67 (2), 93–99. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Madden, S.; Back, D. J. Metabolism of Norgestimate by Human Gastrointestinal Mucosa and Liver Microsomes in Vitro. J. Steroid Biochem. Mol. Biol. 1991, 38 (4), 497–503. (View all citations for this reference)
- Wong, F. A.; Edom, R. W.; Duda, M.; Tischio, J. P.; Huang, M.; Juzwin, S.; Tegegne, G. Determination of Norgestimate and Its Metabolites in Human Serum Using High-Performance Liquid Chromatography with Tandem Mass Spectrometric Detection. J. Chromatogr. B Biomed. Sci. Appl. 1999, 734 (2), 247–256. (View all citations for this reference)
- MonoNessa: Norgestimate and Ethinyl Estradiol. DailyMed, a National Library of Medicines online database. (View all citations for this reference)
- White, T.; Özel, B.; Jain, J. K.; Stanczyk, F. Z. Effects of Transdermal and Oral Contraceptives on Estrogen-Sensitive Hepatic Proteins. Contraception 2006, 74 (4), 293–296. (View all citations for this reference)
- FDA Ortho Evra Information (View all citations for this reference)