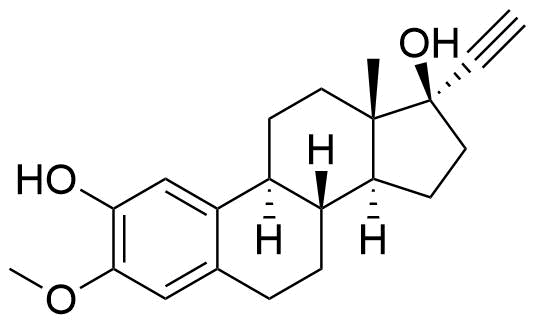
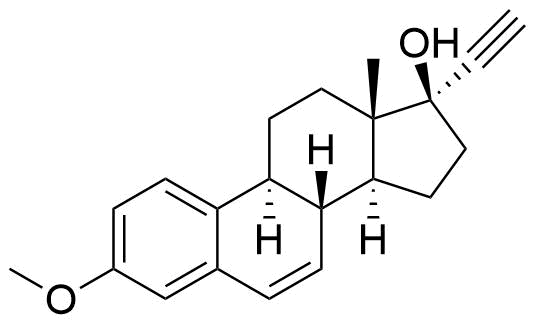
Mestranol
Mestranol (EEME) is a synthetic steroidal estrogen and a biologically inactive prodrug of ethinyl estradiol. It is used alongside progestins in combined oral contraceptives and hormone replacement therapy, although it has largely been replaced by ethinyl estradiol in the last several decades.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Ethinyl Estradiol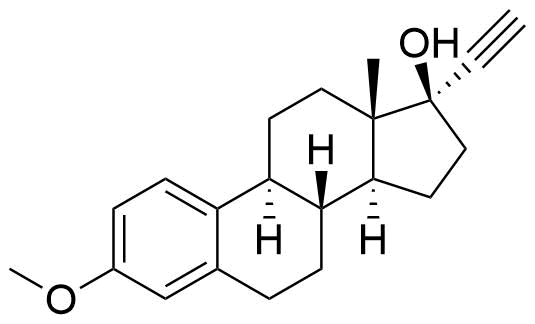
Identifiers
Abbreviation
EEME
References
Names
- ethinyl estradiol 3-methyl-ether
- 17α-ethinyl-3-(methyloxy)estra-1,3,5(10)-trien-17β-ol
- 3-methoxy-19-nor-17α-pregna-1,4,5(10)-trien-20-yn-17-ol
- CB-8027
- L-33355
- RS-1044
References
CASRN
72-33-3
References
PubChem CID
6291
ECHA InfoCard
- 100.000.707
- EC / List #: 200-777-8
IUPHAR/BPS
7087
DrugBank Accession Number
DB01357
References
- DrugBank: Mestranol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
B2V233XGE7
KEGG Entry Number
D00575
Wikipedia Entry Name
Mestranol
ChEBI ID
CHEBI:6784
ChEMBL ID
CHEMBL1201151
ChemSpider ID
6054
NIST
Mestranol
Physical & Chemical Properties
Molecular Formula
C21H26O2
References
Molecular Weight
310.43 g/mol
References
Appearance
White or almost white crystalline powder
References
- British Pharmacopoeia 2017: Mestranol monograph (View all citations for this reference)
Melting Point
ChemIDPlus, Toxnet: 150.5° C
USP: 146-154°, range between beginning and end of melting not larger than 4°
BP: 150-154° C
References
- ChemIDPlus: A Toxnet Database. Mestranol. (View all citations for this reference)
- USP 40: Mestranol monograph. (View all citations for this reference)
- British Pharmacopoeia 2017: Mestranol monograph (View all citations for this reference)
- Toxnet: Mestranol. (View all citations for this reference)
Solubility
Practically insoluble in water, sparingly soluble in alcohol
References
- British Pharmacopoeia 2017: Mestranol monograph (View all citations for this reference)
Specific Optical Rotation
+2° to +8°, 20 mg dried per mL dioxane
References
- USP 40: Mestranol monograph. (View all citations for this reference)
Storage Conditions
Store at 25 °C or below out of direct sunlight.
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Skin Corrosion/Irritation | 2 | H315 | Causes skin irritation |
| Serious Eye Damage/Irritation | 2 | H319 | Causes serious eye irritation |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360FD | May damage fertility. May damage the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
| Mutagenicity | 3 | H341 | Suspected of causing genetic defects |
Genotoxicity
Shown to be genotoxic to human lymphocytes and mouse bone-marrow cells through chromosome aberrations and sister chromatid exchanges. Ames Salmonella/S9 assay with and without S9 mix and host-mediated assay results were negative.
LD50
mouse intraperitoneal: > 3.2 g/kg
mouse oral: > 10 g/kg
mouse subcutaneous: 2.5 g/kg
rat intraperitoneal: > 3 g/kg
rat oral: > 10 g/kg
rat subcutaneous: > 5 g/kg
TD50
mouse: 0.279 mg/kg/day (In Enovid (mestranol/norethynodrel) formulation)
MRTD
0.00083 mg/kg/day
Biochemistry & Pharmacology
Estrogen Receptor Activity
Agonist, but binds poorly. Most of its effects are carried out via metabolism to ethinyl estradiol.
References
- ChEBI: Mestranol (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Mestranol. (View all citations for this reference)
Metabolism
Hepatic, metabolized to ethinyl estradiol.
Bioequivalence
70% (50 μg of mestranol is pharmacologically bioequivalent to 35-40 μg ethinyl estradiol).
Metabolites
Name
Structure
Notes
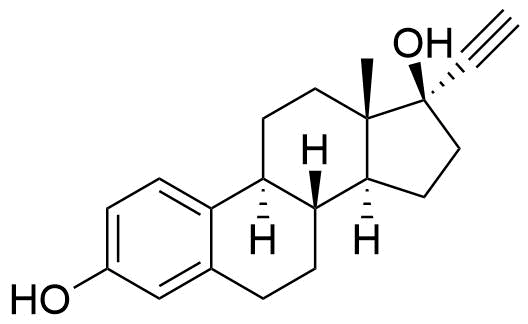
Primary metabolite of mestranol and quinestrol. Also a minor metabolite of norethindrone (0.7 and 1.0% of norethindrone converted to ethinyl estradiol at doses of 5 and 10 mg, respectively).