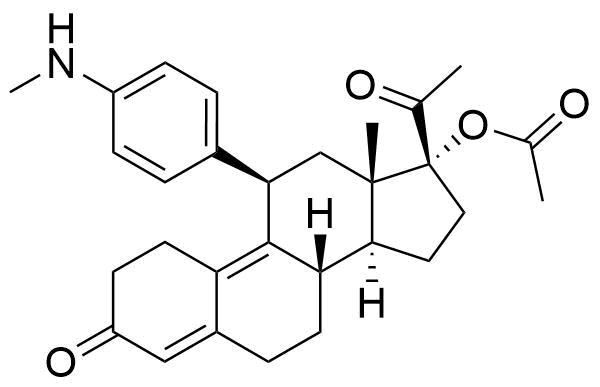
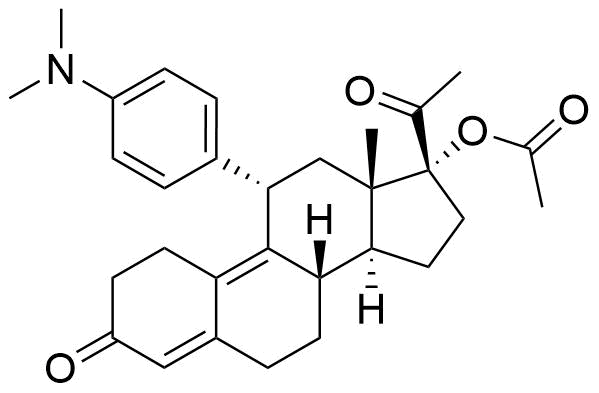
Ulipristal Acetate
Ulipristal acetate (ULIPA) is a synthetic progesterone receptor modulator (SPRM) used in emergency hormonal contraceptives and for treatment of uterine fibroids.
Tags
Approvals
WHO Essential Medicine US FDA-Approved
Identifiers
Abbreviation
ULIPA
References
Names
- 17α-acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20- dione
- CDB(VA)-2914
References
CASRN
126784-99-4
References
PubChem CID
130904
IUPHAR/BPS
7460
DrugBank Accession Number
DB08867 (Ulipristal)
References
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
YF7V70N02B
KEGG Entry Number
D09687
Wikipedia Entry Name
Ulipristal Acetate
ChEBI ID
CHEBI:71025
ChEMBL ID
CHEMBL260538
ChemSpider ID
115762
ATC Code(s)
References
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C30H37NO4
References
Molecular Weight
475.62 g/mol
References
Appearance
White to yellow crystalline powder
Solubility
Freely soluble in CHCl3, soluble in methanol, acetone, ethanol, and insoluble in water.
Polymorphism
Two found
Toxicology
Side Effects
Headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.
References
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Ulipristal acetate (ella) National Drug Monograph. October 2014. VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. (View all citations for this reference)
- Side Effects Database, Ulipristal. (View all citations for this reference)
Genotoxicity
"No genotoxic potential was evident in any of the test systems when tested up to appropriate concentrations and dose levels according to guidelines."
LD50
< 1250 mg/kg (rat), >1250 mg/kg (rabbit)
Biochemistry & Pharmacology
Progesterone Receptor Activity
Partial agonist and antagonist
References
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Zhao, Y.; Li, X.; Liu, H.; Yu, Y.; Hai, L.; Guo, L.; Wu, Y., First synthesis and characterization for the stereoisomers of Ulipristal acetate. Steroids 2015, 95, 7-16. (View all citations for this reference)
Androgen Receptor Activity
No activity
References
- Attardi, B. J.; Burgenson, J.; Hild, S. A.; Reel, J. R., In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol 2004, 88 (3), 277-88. (View all citations for this reference)
Estrogen Receptor Activity
No activity
References
- Attardi, B. J.; Burgenson, J.; Hild, S. A.; Reel, J. R., In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol 2004, 88 (3), 277-88. (View all citations for this reference)
Glucocorticoid Receptor Activity
Antagonist
References
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Zhao, Y.; Li, X.; Liu, H.; Yu, Y.; Hai, L.; Guo, L.; Wu, Y., First synthesis and characterization for the stereoisomers of Ulipristal acetate. Steroids 2015, 95, 7-16. (View all citations for this reference)
Mineralocorticoid Receptor Activity
No activity
References
- Attardi, B. J.; Burgenson, J.; Hild, S. A.; Reel, J. R., In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol 2004, 88 (3), 277-88. (View all citations for this reference)
Elimination Half-Life (t1/2)
32.4 +/- 6.3 h (from 30 mg oral dose)
References
- 30 mg ulipristal acetate tablet for emergency contraception. Laboratoire HRA Pharma, Distributed by: Actavis Specialty Pharmaceuticals Co. January 22, 2015. (View all citations for this reference)
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Serum Protein Binding
>94% bound to plasma proteins, including high density lipoprotein, alpha-l-acid glycoprotein, and albumin
Metabolism
Metabolized by CYP3A4 and to a lesser extent by CYP1A2
References
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Excretion
Mostly eliminated in feces, <10% in urine. Small amounts excreted in breast milk.
References
- 30 mg ulipristal acetate tablet for emergency contraception. Laboratoire HRA Pharma, Distributed by: Actavis Specialty Pharmaceuticals Co. January 22, 2015. (View all citations for this reference)
- Product Monograph: Fibristal, 5 mg ulipristal acetate tablet. Allergan Pharma Co. Nov. 23, 2016. (View all citations for this reference)
Cmax
176 +/- 89 ng/mL (healthy subjects, single oral dose)
References
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Tmax
0.9 h
AUC
556 +/- 260 ng*h/mL (0-∞, healthy subjects, single oral dose)
References
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Clearance
76.8 +/- 64.0 L/h (mean oral clearance, single oral dose, health subject)
References
- DrugBank: Ulipristal
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Permeability Glycoprotein (P-gp) Binding
Does not appear to be a P-gp substrate
Metabolites
Name
Structure
Notes
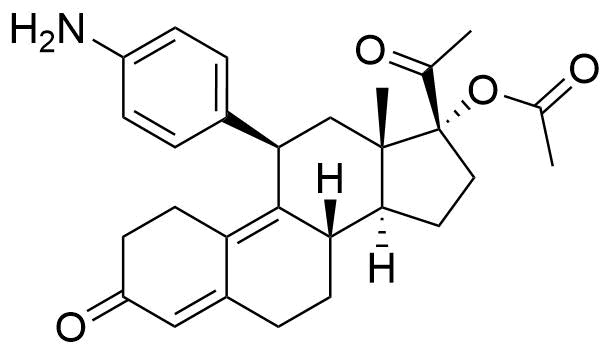
Not pharmacologically active. Also referred to as PGL4004. Conversion from ulipristal acetate most likely mediated by CYP3A4.
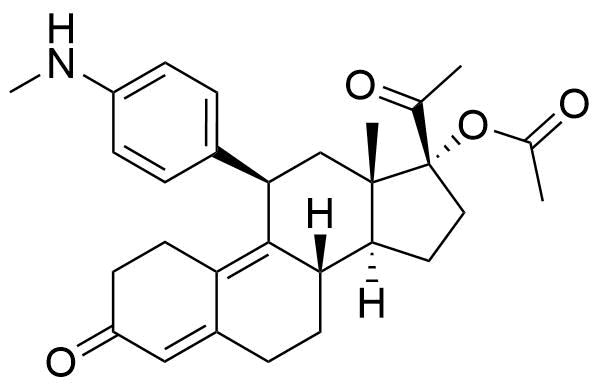
Also known as metabolite PGL4002. Pharmacologically active. Binds to human plasma proteins extensively at 96.5%. Most likely mediated by CYP3A4.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
US FDA-Approved Products
WHO Essential Medicines
Name
Formulation
Tablet