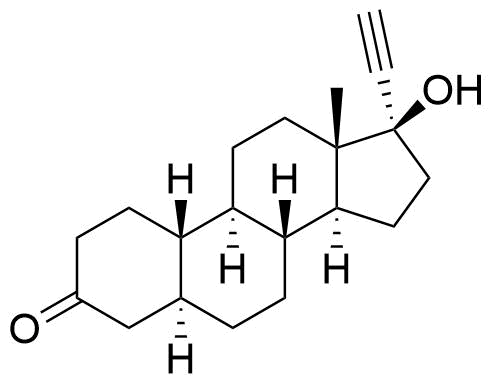
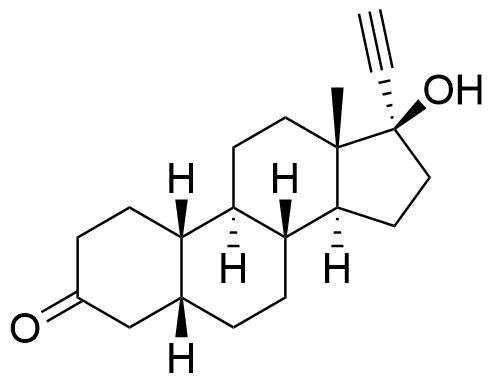
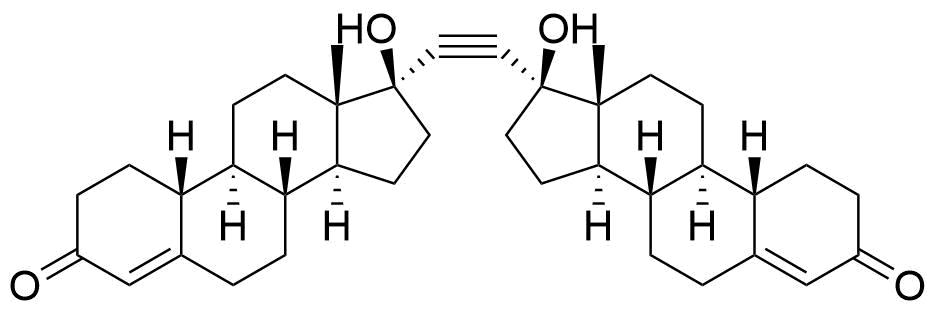
Norethindrone
Norethindrone (NET), or norethisterone, is a synthetic progestogen derived from testosterone and used in hormonal contraceptives, hormone replacement therapy, and treatment of gynecological disorders. Prodrugs of NET used in medications include norethindrone acetate (NETA) and norethindrone enanthate (NETE).
Tags
Approvals
WHO PrequalificationRelated Compounds
Norethindrone Acetate Norethindrone Enanthate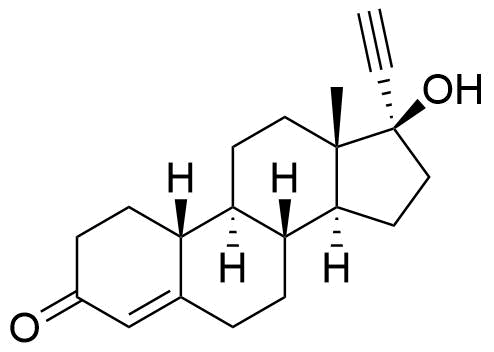
Identifiers
Abbreviation
NET
References
Names
- norethisterone
- 17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one
- ethinylnortestosterone
References
CASRN
68-22-4
References
PubChem CID
6230
ECHA InfoCard
- 100.000.619
- EC / List #: 200-681-4
IUPHAR/BPS
2880
DrugBank Accession Number
DB00717
References
- DrugBank: Norethisterone
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
T18F433X4S
KEGG Entry Number
D00182
Wikipedia Entry Name
Norethisterone
ChEBI ID
CHEBI:7627
ChEMBL ID
CHEMBL1162
ChemSpider ID
5994
PDB
NDR
NIST
Norethisterone
ATC Code(s)
Physical & Chemical Properties
Molecular Formula
C20H26O2
References
Molecular Weight
298.419 g/mol
References
Appearance
White or yellowish white, odorless, crystalline powder
References
- British Pharmacopoeia 2014: Norethisterone API (View all citations for this reference)
- British Pharmacopoeia 2017: Norethisterone monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Melting Point
Zafar: 201-203 °C
IARC, Toxnet: 203-204 °C
References
- Zafar, S.; Yousuf, S.; Kayani, H. A.; Saifullah, S.; Khan, S.; Al-Majid, A. M.; Choudhary, M. I. Biotransformation of Oral Contraceptive Ethynodiol Diacetate with Microbial and Plant Cell Cultures. Chem. Cent. J. 2012, 6 (1), 452. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Norethindrone. (View all citations for this reference)
Solubility
Insoluble in water, soluble in chloroform and dioxane, sparingly soluble in acetone and anhydrous ethanol. Slightly soluble in diethyl ether.
References
- British Pharmacopoeia 2014: Norethisterone API (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
logP
2.97
Specific Optical Rotation
-32.0 to -37.0 (dried)
-31.7 °C in chloroform
References
- British Pharmacopoeia 2014: Norethisterone API (View all citations for this reference)
- British Pharmacopoeia 2017: Norethisterone monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Storage Conditions
Stable in air, unstable when exposed to both air and light.
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360FD | May damage fertility. May damage the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
Mutagenicity
Not found to be mutagenic in the Ames Salmonella/microsome direct plate incorporation protocol.
References
- Lang, R.; Reimann, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. I. Communication: Examination for the Induction of Gene Mutations Using the Ames Salmonella/microsome Test and the HGPRT Test in V79 Cells. Environ. Mol. Mutagen. 1993, 21 (3), 272–304. (View all citations for this reference)
Genotoxicity
Reports vary: Ahmad et al. reported it as non-genotoxic to human lymphocyte chromosomes as measured by chromosomal aberrations, sister chromatid exchanges, and cell growth kinetics. Martelli et al. suggested it could be a week genotoxin.
References
- Ahmad, M. E.; Shadab, G.; Azfer, M. A.; Afzal, M. Evaluation of Genotoxic Potential of Synthetic Progestins-Norethindrone and Norgestrel in Human Lymphocytes in Vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2001, 494 (1–2), 13–20. (View all citations for this reference)
- Martelli, A.; Mereto, E.; Ghia, M.; Orsi, P.; Allavena, A.; De Pascalis, C. R.; Brambilla, G. Induction of Micronuclei and of Enzyme-Altered Foci in the Liver of Female Rats Exposed to Progesterone and Three Synthetic Progestins. Mutat. Res. Toxicol. Environ. Mutagen. 1998, 419 (1–3), 33–41. (View all citations for this reference)
LD50
LD50, mouse oral: 6 g/kg
TDLo, human women oral: 42 mg/kg
MRTD
0.0167 mg/kg/day
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist
References
Androgen Receptor Activity
Agonist
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
Estrogen Receptor Activity
Agonist and antagonist
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
Glucocorticoid Receptor Activity
No activity
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
Mineralocorticoid Receptor Activity
No activity
References
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
Target Pathways
Bioavailability
64%
Elimination Half-Life (t1/2)
7-8 h (Stanczyk, Goodman & Gilman)
4.8 - 12.8 h (Toxnet)
References
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Toxnet: Norethindrone. (View all citations for this reference)
Serum Protein Binding
> 95%, 61% to albumin, 36% to SHBG
References
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Nilsson, B.; von Schoultz, B., Binding of levonorgestrel, norethisterone and desogestrel to human sex hormone binding globulin and influence on free testosterone levels. Gynecol Obstet Invest 1989, 27 (3), 151-4. (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Toxnet: Norethindrone. (View all citations for this reference)
Metabolism
CYP3A4
Apparent Volume of Distribution
4 L/kg
Clearance
0.4 L/h/kg
Enzyme Interactions
Estrone sulfatase inhibitor
Inhibition of Ovulation
0.5 mg/day
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Transformation of Endometrium
100-150 mg/cycle
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Menstrual Delay
10-15 mg/day
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Indications
Menopause
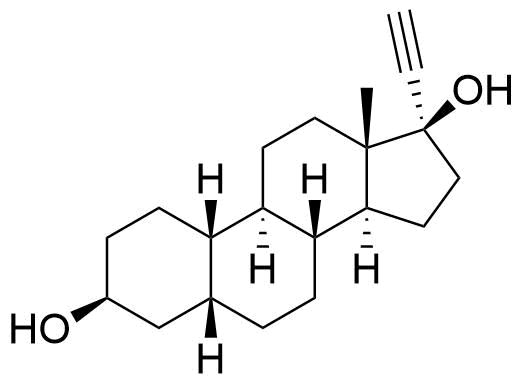
Metabolites
Name
Structure
Notes
Sulfate and glucuronide conjugates of norethindrone and its metabolites are also present.
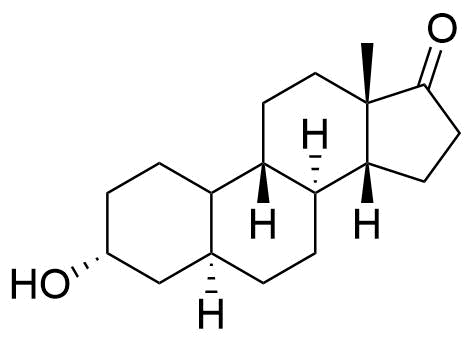
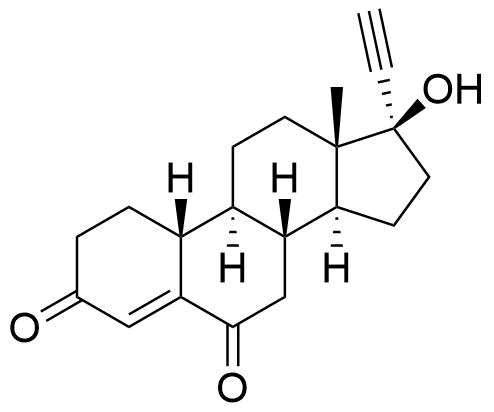
The epimer 19-noretiocholanolone may also be formed by metabolism of NET. Detection of 19-norandrosterone in an athlete's urine is taken as evidence of doping by nandrolone or another 19-steroid. However, this compound is a metabolite of norethindrone, and as a metabolite, it can be detected in quantities above the reporting threshold of 2 ng/mL.
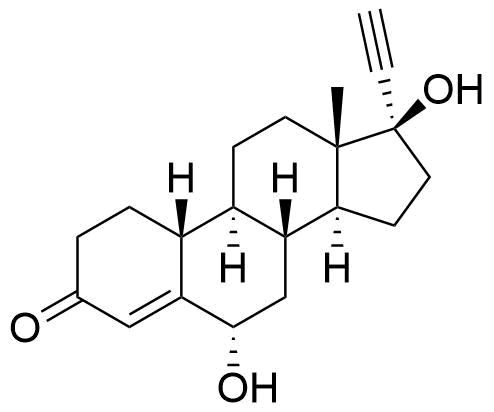
Impurities
Name
Structure
CASRN
Other Names & Identifiers
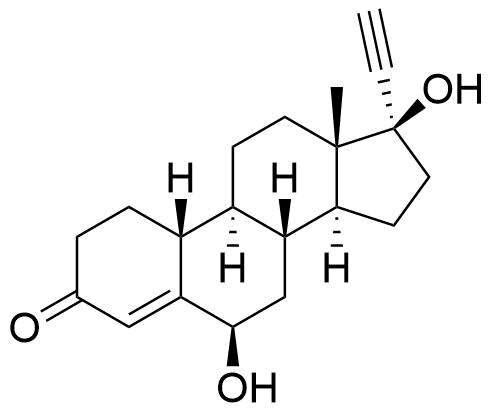
- BP Norethisterone Impurity H
- 6β,17-dihydroxy-19-nor-17α-pregn-4-en-20-yn-3-one
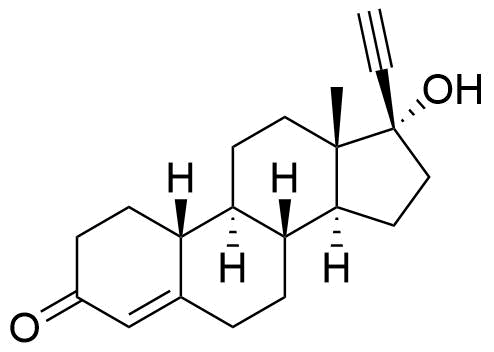
- BP Norethisterone Impurity G
- 17-hydroxy-19-norpregn-4-en-20-yn-3-one
- Impurity 3 (Gorog 1987)
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Prescription
018977
Discontinued
018977
NORETHINDRONE: 1 mg, 1 mg, 1 mg
28 oral tablets
Prescription
076105