Norethindrone Enanthate
Norethindrone enanthate (NETE), or norethisterone enanthate, is an ester prodrug of norethindrone. It is a synthetic progestogen used in place of norethindrone in some hormonal contraceptives.
Tags
Approvals
WHO Essential Medicine WHO PrequalificationRelated Compounds
Norethindrone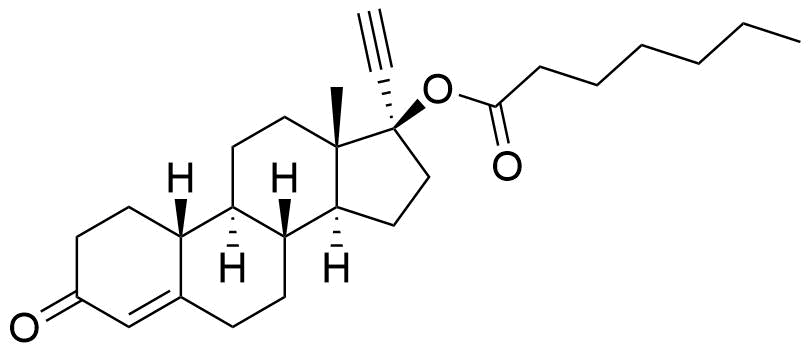
Identifiers
Abbreviation
NETE
References
Names
- norethisterone enanthate
- norethindrone enantate
- 17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one heptanoate
- 17-[(1-oxoheptyl)oxy]-19-nor-17α-pregn-4-en-20- yn-3-one
References
CASRN
3836-23-5
References
PubChem CID
229295
ECHA InfoCard
- 100.021.207
- EC / List #: 223-326-7
UNII
HY3S2K0J0F
Wikipedia Entry Name
Norethisterone Enanthate
ChEBI ID
CHEBI:34894
ChemSpider ID
199613
NIST
Norethisterone Enanthate
Physical & Chemical Properties
Molecular Formula
C27H38O3
References
Molecular Weight
410.6 g/mol
References
Appearance
White to creamy white crystalline powder. No odor.
Melting Point
68-73 °C
Specific Optical Rotation
[α]D20 °C = -10.0° to -15.0° in 20 mg/mL solution chloroform
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360 | May damage fertility or the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
Mutagenicity
Not found to be mutagenic in the Ames Salmonella/microsome direct plate incorporation protocol.
References
- Lang, R.; Reimann, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. I. Communication: Examination for the Induction of Gene Mutations Using the Ames Salmonella/microsome Test and the HGPRT Test in V79 Cells. Environ. Mol. Mutagen. 1993, 21 (3), 272–304. (View all citations for this reference)
Toxicology
See norethindrone.
References
Biochemistry & Pharmacology
Pharmacology
See norethindrone
References
Metabolites
Name
Structure
Notes
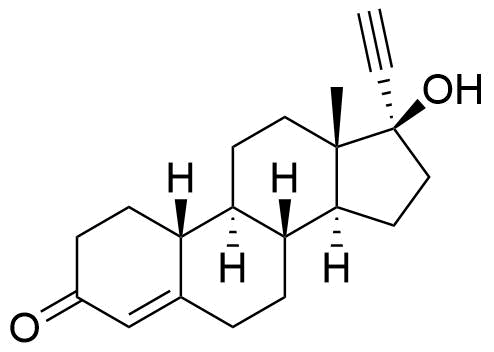
Norethindrone acetate, norethindrone enanthate, lynestrenol, and ethynodiol diacetate (and to a very small extent, norethynodrel) are prodrugs of norethindrone. Conversion of lynestrenol to ethynodiol to norethindrone facilitated by CYP2C9, CYP2C19, and CYP3A4. Conversion of norethynodrel to norethindrone is accomplished by either non-enzymatic or enzymatic ketosteroid isomerization.
WHO Essential Medicines
Name
Formulation
[Listed as NORETHISTERONE ENANTATE]
Oily solution injection
WHO Prequalified Medicines
WHO Reference #
Name
Applicant
Formulation
[Listed as NORETHISTERONE ENANTATE]