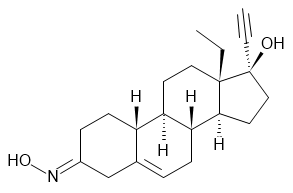
Norelgestromin
Norelgestromin (NGMN) is a synthetic progestogen used in combined oral contraceptives and in menopausal hormonal therapy. It is the primary metabolite of norgestimate.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Norgestimate
Identifiers
Abbreviation
NGMN
References
Names
- levonorgestrel 3-oxime
- 17β-deacetylnorgestimate
- 17α-ethynyl-18-methyl-19-nortestosterone 3-oxime
- 17α-ethynyl-18-methylestr-4-en-17β-ol-3-one 3-oxime
- 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, oxime, (17α)-
- 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one oxime
References
CASRN
53016-31-2
References
PubChem CID
62930
DrugBank Accession Number
DB06713
References
- DrugBank: Norelgestromin
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
R0TAY3X631
KEGG Entry Number
D05205
Wikipedia Entry Name
Norelgestromin
ChEBI ID
CHEBI:135398
ChEMBL ID
CHEMBL1200807
ChemSpider ID
56648
ATC Code(s)
References
- DrugBank: Norelgestromin
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C21H29NO2
References
Molecular Weight
327.46 g/mol
References
Specific Optical Rotation
+35° to +41 °, 5 mg/mL in 75:25 alcohol:water
References
- USP 40: Norelgestromin monograph. (View all citations for this reference)
Ratio of (E)- to (Z)- Isomer
Between 1.3 and 1.6
References
- USP 40: Norelgestromin monograph. (View all citations for this reference)
Biochemistry & Pharmacology
Progesterone Receptor Activity
Partial agonist
References
- DrugBank: Norelgestromin
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Paris, F.; Balaguer, P.; Rimbault, F.; Gaspari, L.; Sultan, C. Molecular Action of Norgestimate: New Developments. Gynecol. Endocrinol. 2015, 31 (6), 487–490. (View all citations for this reference)
Androgen Receptor Activity
Partial agonist
References
- DrugBank: Norelgestromin
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Estrogen Receptor Activity
Selective agonist for ERα
Glucocorticoid Receptor Activity
Antagonist with low affinity
Mineralocorticoid Receptor Activity
Antagonist with moderate affinity
Elimination Half-Life (t1/2)
- 29.2 +/- 14.5 h from IV injection of 252 μg norelgestromin and 25 μg ethinyl estradiol.
- 28.4 +/- 12.8 h from transdermal patch of 150 μg/day norelgestromin and 20 μg/day ethinyl estradiol.
References
- Abrams, L. S.; Skee, D.; Natarajan, J.; Wong, F. A. Pharmacokinetic Overview of Ortho Evra (TM)/Evra (TM). Fertil. Steril. 2002, 77 (2, 2), S3–S12. (View all citations for this reference)
Serum Protein Binding
>97% bound, primarily to albumin. No binding to SHBG, except as metabolite norgestrel.
References
- Abrams, L. S.; Skee, D.; Natarajan, J.; Wong, F. A. Pharmacokinetic Overview of Ortho Evra (TM)/Evra (TM). Fertil. Steril. 2002, 77 (2, 2), S3–S12. (View all citations for this reference)
- Toxnet: Norgestimate. (View all citations for this reference)
- FDA Ortho Evra Information (View all citations for this reference)
Metabolism
CYP3A4
Excretion
Via renal and fecal pathways.
Clearance
7.89 +/- 1.63 L/h from IV injection of 252 μg norelgestromin and 25 μg ethinyl estradiol.
References
- Abrams, L. S.; Skee, D.; Natarajan, J.; Wong, F. A. Pharmacokinetic Overview of Ortho Evra (TM)/Evra (TM). Fertil. Steril. 2002, 77 (2, 2), S3–S12. (View all citations for this reference)
Enzyme Interactions
Inhibits estrone sulfatase
References
- DrugBank: Norelgestromin
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Pasqualini, J. R. The Selective Estrogen Enzyme Modulators in Breast Cancer: A Review. Biochim. Biophys. Acta - Rev. Cancer 2004, 1654 (2), 123–143. (View all citations for this reference)
- Pasqualini, J. R.; Caubel, P.; Friedman, A. J.; Philippe, J.-C.; Chetrite, G. S. Norelgestromin as Selective Estrogen Enzyme Modulator in Human Breast Cancer Cell Lines. J. Steroid Biochem. Mol. Biol. 2003, 84 (2–3), 193–198. (View all citations for this reference)
Metabolites
Name
Structure
Notes
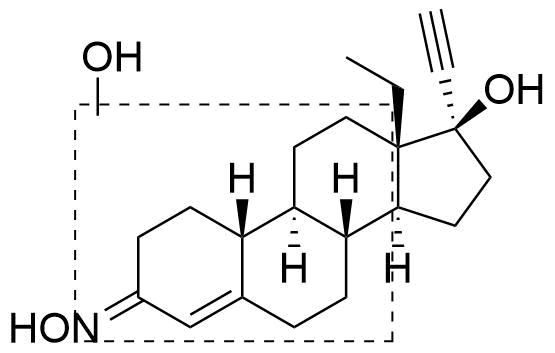
Position of hydroxyl group not specified. Referred to as Met6 in source.
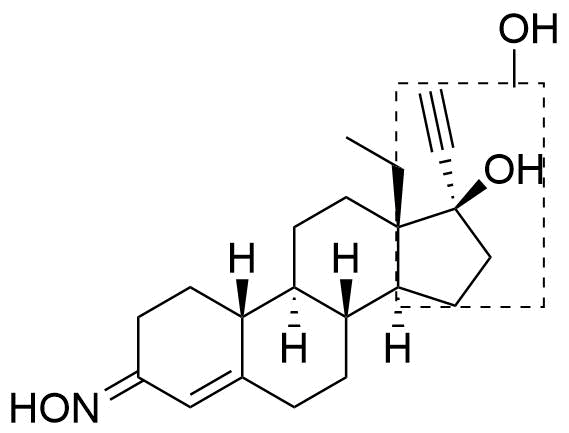
Position of hydroxyl group not specified. Referred to as Met5 in source.
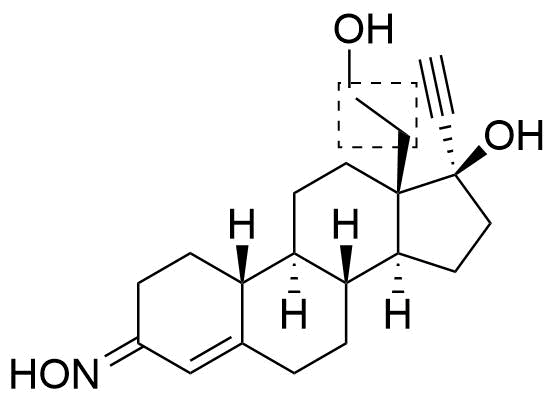
Position of hydroxyl group not specified. Referred to as Met4 in source.
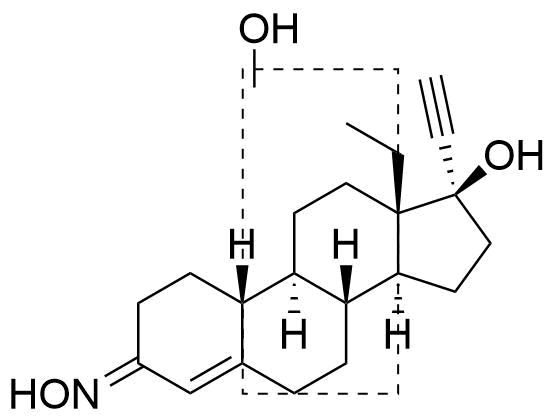
Position of hydroxyl group not specified. Referred to as Met3 in source.
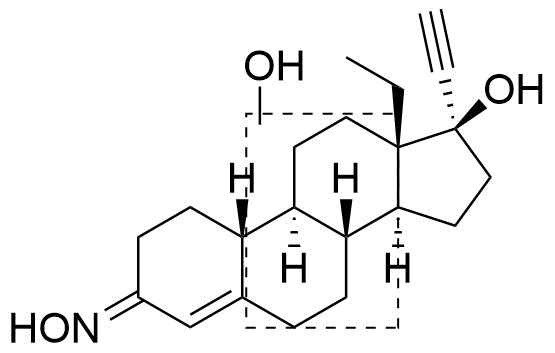
Position of hydroxyl group not specified. Referred to as Met1 in source.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
US FDA-Approved Products
Name
Formulation
Status
ANDA #
NORELGESTROMIN: 0.15 mg/day
Film, extended release; transdermal
Prescription
200910
NORELGESTROMIN: 0.15 mg/day
Film, extended release; transdermal
Discontinued
021180