Ormeloxifene
Ormeloxifene, or centchroman, is a selective estrogen receptor modulator (SERM), used as a non-hormonal contraceptive in India.
Tags
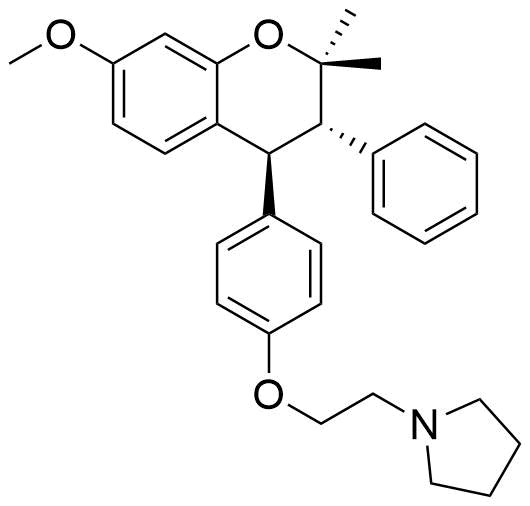
Identifiers
Names
Centchroman
CASRN
78994-24-8
PubChem CID
154413
UNII
44AXY5VE90
KEGG Entry Number
D08301
Wikipedia Entry Name
Ormeloxifene
ATC Code(s)
Physical & Chemical Properties
Molecular Formula
C30H35NO3
References
Molecular Weight
457.604 g/mol
References
Appearance
white crystalline substance
Melting Point
165-166 °C
Solubility
Soluble in chloroform, acetone, methanol, and ethanol. Almost insoluble in water, isobutanol, HCl, or NaOH.
logP
7.41
Storage Conditions
Highly stable under normal storage conditions
References
Toxicology
Side Effects
Nausea, headache, weight gain, delayed or prolonged menstrual period.
References
- Anjum, S.; Agrawal, A.; Kulshreshtha, S.; Sharma, R.; Namita, N. A Study of Efficacy of Ormeloxifene in the Pharmacological Management of Dysfunctional Uterine Bleeding. J. Evol. Med. Dent. Sci. 2015, 4 (73), 12639–12644. (View all citations for this reference)
Carcinogenicity
No carcinogenic effects observed in rats and mice
Mutagenicity
Lacks mutagenicity
Genotoxicity
Lacks genotoxicity
LD50
Mouse, oral, both D and L enantiomers: >1600 mg/kg
Mouse, intraperitoneal, both D and L enantiomers: 400 mg/kg
Mouse, intraperitoneal, L enantiomer: 316 mg/kg
Mouse, intraperitoneal, D enantiomer: 383 mg/kg
Rat, oral, both D and L enantiomers: >2000 mg/kg
Rhesus monkey, intraperitoneal, both D and L enantiomers: 216 mg/kg
Pharmacology
Androgen Receptor Activity
No activity
Estrogen Receptor Activity
Competitive partial agonist: has both agonist and antagonist behavior, depending on the tissue it's in.
References
- Wikipedia: Ormeloxifene. (View all citations for this reference)
- Anjum, S.; Agrawal, A.; Kulshreshtha, S.; Sharma, R.; Namita, N. A Study of Efficacy of Ormeloxifene in the Pharmacological Management of Dysfunctional Uterine Bleeding. J. Evol. Med. Dent. Sci. 2015, 4 (73), 12639–12644. (View all citations for this reference)
- Singh, M. M. Centchroman, a Selective Estrogen Receptor Modulator, as a Contraceptive and for the Management of Hormone-Related Clinical Disorders. Med. Res. Rev. 2001, 21 (4), 302–347. (View all citations for this reference)
Elimination Half-Life (t1/2)
Human women: 168 h
Rhesus monkey: 45 h
Rat: 24.1 h
Serum Protein Binding
Binds to albumin
Excretion
Primarily through feces. Excreted in milk, but "unlikely to be of much physiological consequence to suckling babies."
Cmax
122.57 +/- 6.25 ng/mL from single 60 mg oral dose
55.25 +/1 15.45 ng/mL from single 30 mg oral dose
67.55 +/- 6.84 ng/mL from single 30 mg dose of Saheli product
69.44 +/- 9.38 ng/mL from single 30 mg dose of Centron product
54.98 +/- 14.19 ng/mL from 30 mg dose twice a week for 12 weeks
Tmax
4 h from single 60 mg oral dose
5.18 +/- 1.78 h from single 30 mg oral dose
5.33 +/- 0.84 h from single 30 mg oral dose of Saheli product
4.33 +/- 0.67 h from single 30 mg oral dose of Centron product
6.637 +/- 1.15 h from 30 mg oral dose twice a week for 12 weeks
Indications
Oral contraception, dysfunctional uterine bleeding, advanced breast cancer.
References
- Anjum, S.; Agrawal, A.; Kulshreshtha, S.; Sharma, R.; Namita, N. A Study of Efficacy of Ormeloxifene in the Pharmacological Management of Dysfunctional Uterine Bleeding. J. Evol. Med. Dent. Sci. 2015, 4 (73), 12639–12644. (View all citations for this reference)
Metabolites
Name
Structure
Notes
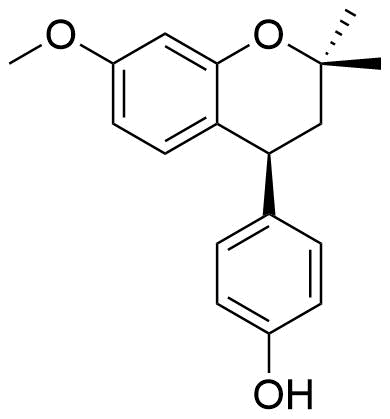
Identified as metabolite from rat liver homogenate. 39.4% of all metabolites identified from this source.
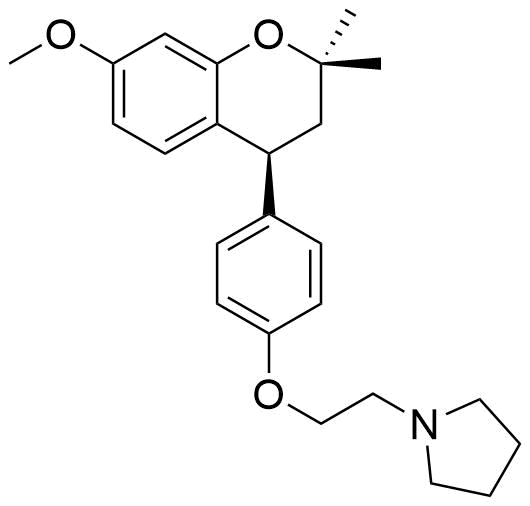
Identified as metabolite from rat liver homogenate. 8.8% of all metabolites identified from this source.
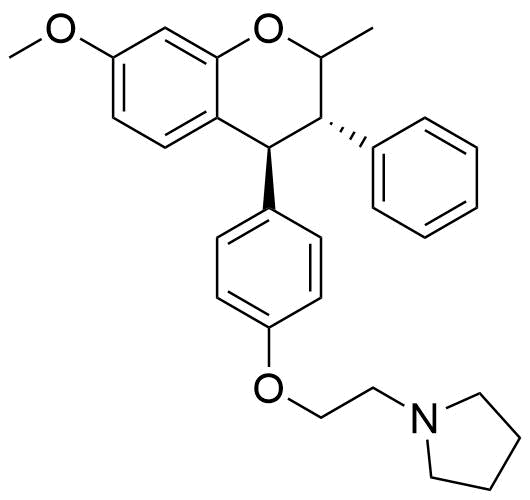
Identified as metabolite from rat liver homogenate. 2.9% of all metabolites identified from this source.
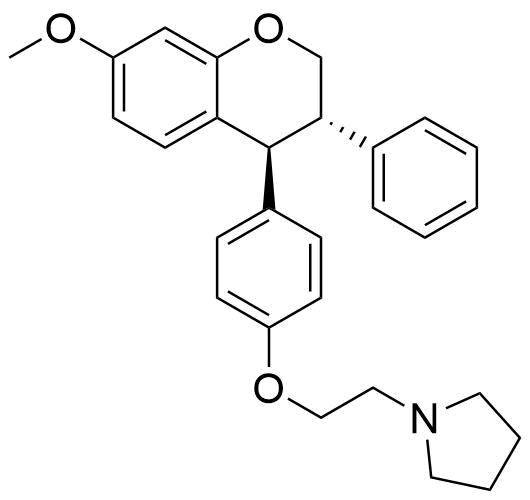
Identified as metabolite from rat liver homogenate. 37.5% of all metabolites identified from this source.
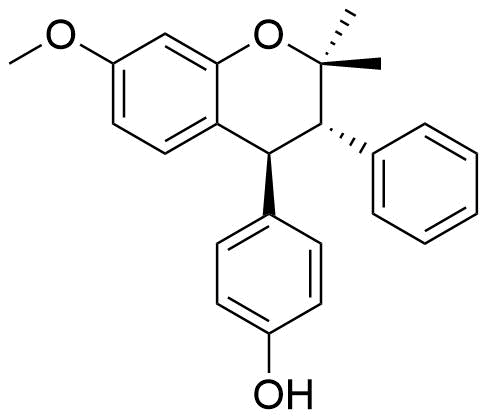
Identified as metabolite from rat liver homogenate. 5.8% of all metabolites identified from this source.