| Other Ethynodiol Diacetate Metabolites |
|
As ethynodiol diacetate is a prodrug of norethindrone, metabolites of norethindrone are formed after administration of ethynodiol diacetate. These metabolites include all 4 isomers of 3,5-tetrahydronorethindrone: 3α,5α-, 3β,5α, 3α,5β, and 3β,5β. |
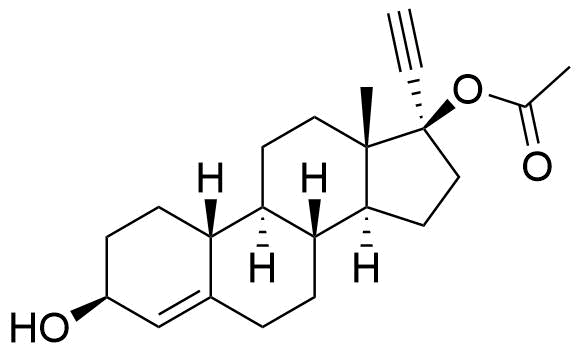
| 3β-Hydroxynorethindrone Acetate |
|
Reported as in vitro metabolite of ethynodiol diacetate by human liver cells. |
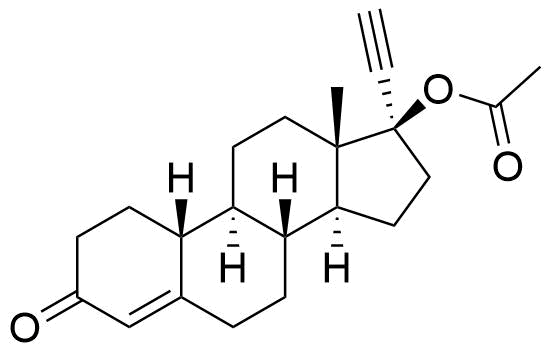
| Norethindrone Acetate |
|
Reported as in vitro metabolite of ethynodiol diacetate by human liver cells. |
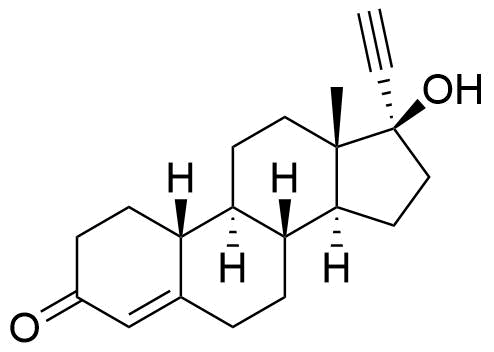
| Norethindrone |
|
Norethindrone acetate, norethindrone enanthate, lynestrenol, and ethynodiol diacetate (and to a very small extent, norethynodrel) are prodrugs of norethindrone. Conversion of lynestrenol to ethynodiol to norethindrone facilitated by CYP2C9, CYP2C19, and CYP3A4. Conversion of norethynodrel to norethindrone is accomplished by either non-enzymatic or enzymatic ketosteroid isomerization. |


