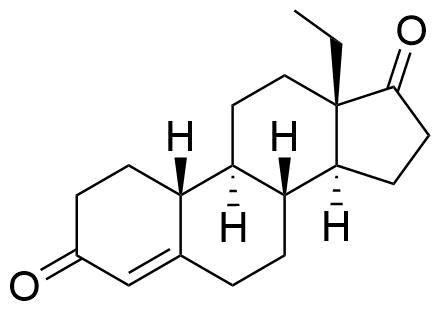
13-Ethylgon-4-ene-3,17-dione
Structure
Other Names & Identifiers
- BP Levonorgestrel Impurity L
CASRN
Notes
BP 2017/EP 8.2 for Levonorgestrel says this impurity is also called levodione; however, sources such as PubChem and ChEBI demonstrate that the name levodione actually applies to a non-steroidal cyclohexane derivative.
Molecular Formula
C19H26O2
References
Molecular Weight
286.41 g/mol
References
Reaxys ID Number
2221392
References
Structural Difference from API
hydroxy & ethynyl groups on C17 replaced by carbonyl
References
Optical Rotary Power
97.8-98° at 589 nm in chloroform, 24-25° C
References
- Micheli RAH, Z. G.; Cohen, N.; Parrish, D. R.; Portland, L. A.; Sciamanna, W.; Scott, M. A.; Wehrli, P. A. Total synthesis of optically-active 19-norsteroids - (+)-estr-4-ene-3,17-dione and (+)-13β-ethylgon-4-ene-3,17-dione. Journal of Organic Chemistry. 1975; 40:675-81. (View all citations for this reference)
- Rao PNC, J. W.; Kim, H. K. Preparative chemical methods for aromatization of 19-nor-δ(4)-3-oxosteroids. Steroids. 1994; 59:621-7. (View all citations for this reference)
Melting Point
174.5-175.5° C in acetone
References
- Micheli RAH, Z. G.; Cohen, N.; Parrish, D. R.; Portland, L. A.; Sciamanna, W.; Scott, M. A.; Wehrli, P. A. Total synthesis of optically-active 19-norsteroids - (+)-estr-4-ene-3,17-dione and (+)-13β-ethylgon-4-ene-3,17-dione. Journal of Organic Chemistry. 1975; 40:675-81. (View all citations for this reference)
Spectra
NMR, IR
References
- Rao PNC, J. W.; Kim, H. K. Preparative chemical methods for aromatization of 19-nor-δ(4)-3-oxosteroids. Steroids. 1994; 59:621-7. (View all citations for this reference)
Spectra
UV-Vis Absorption
References
- Micheli RAH, Z. G.; Cohen, N.; Parrish, D. R.; Portland, L. A.; Sciamanna, W.; Scott, M. A.; Wehrli, P. A. Total synthesis of optically-active 19-norsteroids - (+)-estr-4-ene-3,17-dione and (+)-13β-ethylgon-4-ene-3,17-dione. Journal of Organic Chemistry. 1975; 40:675-81. (View all citations for this reference)
Limits
0.1%
References
- British Pharmacopoeia 2017: Levonorgestrel monograph. (View all citations for this reference)