Segesterone
Structure
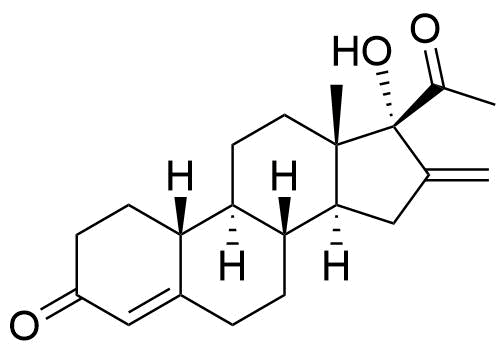
Other Names & Identifiers
- 16-methylene-17α-hydroxy-19-nor-pregn-4-ene-3,20-dione
- 17α-hydroxy-16-methylene-19-norprogesterone
- 17α-deacetylnestorone
CASRN
7690-08-6
Notes
Major degradant. RP-HPLC/MS.
Molecular Weight
328.452 g/mol
References
Structural Difference from Parent
Hydrolysis of 17α acyl group.
library_books
References
- Ahmed, S. M.; Arcuri, F.; Li, F.; Moo-Young, A. J.; Monder, C., Accelerated stability studies on 16-methylene-17α-acetoxy-19-nor-pregn-4-ene-3,20-dione (Nestorone™). Steroids 1995, 60, 534-539. (View all citations for this reference)
Spectra
RP-HPLC/MS
library_books
References
- Ahmed, S. M.; Arcuri, F.; Li, F.; Moo-Young, A. J.; Monder, C., Accelerated stability studies on 16-methylene-17α-acetoxy-19-nor-pregn-4-ene-3,20-dione (Nestorone™). Steroids 1995, 60, 534-539. (View all citations for this reference)
Source
Degradation
library_books
References
- Ahmed, S. M.; Arcuri, F.; Li, F.; Moo-Young, A. J.; Monder, C., Accelerated stability studies on 16-methylene-17α-acetoxy-19-nor-pregn-4-ene-3,20-dione (Nestorone™). Steroids 1995, 60, 534-539. (View all citations for this reference)
References
- Ahmed, S. M.; Arcuri, F.; Li, F.; Moo-Young, A. J.; Monder, C., Accelerated stability studies on 16-methylene-17α-acetoxy-19-nor-pregn-4-ene-3,20-dione (Nestorone™). Steroids 1995, 60, 534-539. (View all citations for this reference)
- Ahmed, S. M. Effect of Cyclodextrins on the Chemical Stability of ST1435, a Contraceptive Steroid Progestin, in Aqueous Solution. J. Incl. Phenom. Mol. Recognit. Chem. 1997, 27 (1), 85–96. (View all citations for this reference)