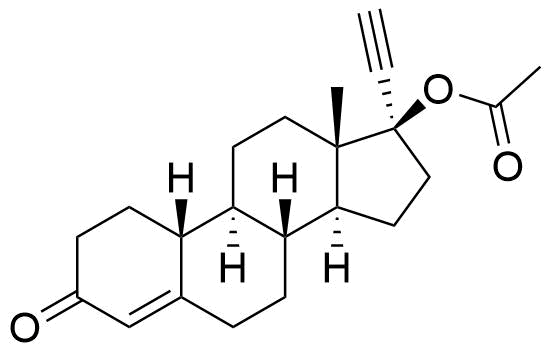
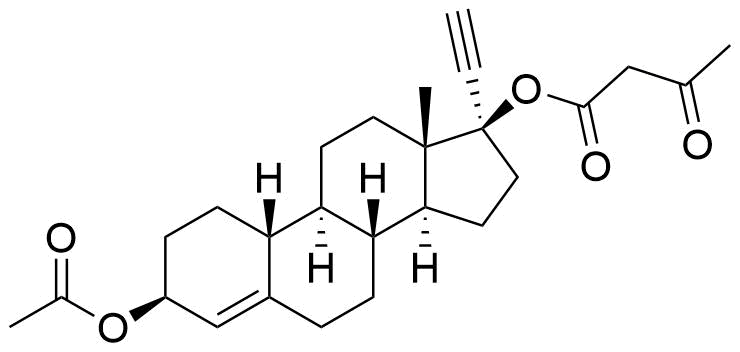
Ethynodiol Diacetate
Ethynodiol diacetate is a synthetic progestogen used in hormonal contraceptives. It is a prodrug of ethynodiol, which is a prodrug of norethindrone.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Norethindrone
Identifiers
Abbreviation
EDA, EDDA
References
Names
- USAN, BAN, JAN: ethynodiol diacetate
- 3β-hydroxynorethisterone 3,17-diacetate
- etynodiol diacetate
- 19-norpregn-4-en-20-yne-3,17-diol, diacetate, (3β,17α)-
- 19-nor-17α-pregn-4-en-20-yne-3β,17-diol diacetate
- 3β-17β-diacetoxy-17α-ethinyl-4-estrene
References
CASRN
297-76-7
References
PubChem CID
92709270
ECHA InfoCard
- 100.005.496
- EC / List #: 206-044-9
DrugBank Accession Number
DB00823
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Wikipedia Entry Name
Etynodiol Diacetate
ChEBI ID
CHEBI:31580
ChEMBL ID
CHEMBL1200624
ChemSpider ID
8913
NIST
Ethynodiol Diacetate
ATC Code(s)
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C24H32O4
References
Molecular Weight
384.509 g/mol
References
Appearance
White, odorless, crystalline powder
Melting Point
126-127 °C
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Solubility
Very slightly soluble to practically insoluble in water, soluble in ethanol, freely to very soluble in chloroform, freely soluble in diethyl ether.
logP
5
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Specific Optical Rotation
-70° to -76°, 10 mg/mL in chloroform
References
- USP 40: Ethynodiol Diacetate monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Storage Conditions
Store between 20 and 25 ° C.
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Acute Dermal Toxicity | 4 | H312 | Harmful in contact with skin |
| Acute Inhalation Toxicity | 4 | H332 | Harmful if inhaled |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
MRTD
0.0167 mg/kg/day
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Estrogen Receptor Activity
Agonist
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Serum Protein Binding
50-85%
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Metabolism
Hepatic. Conversion of ethynodiol diacetate to norethindrone is carried out by esterases.
Indications
Dysfunctional uterine bleeding, dysmenorrhea, hypermenorrhea, polycistic ovarian syndrome
References
- DrugBank: Ethynodiol Diacetate
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Metabolites
Name
Structure
Notes
As ethynodiol diacetate is a prodrug of norethindrone, metabolites of norethindrone are formed after administration of ethynodiol diacetate. These metabolites include all 4 isomers of 3,5-tetrahydronorethindrone: 3α,5α-, 3β,5α, 3α,5β, and 3β,5β.
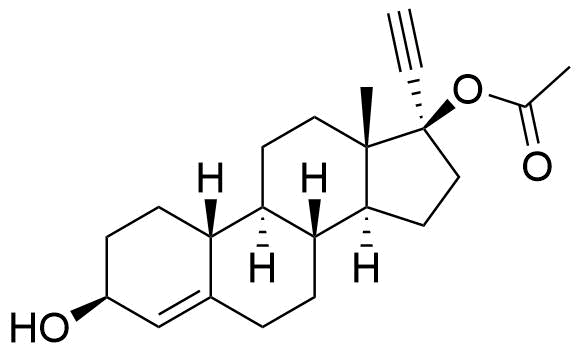
Reported as in vitro metabolite of ethynodiol diacetate by human liver cells.
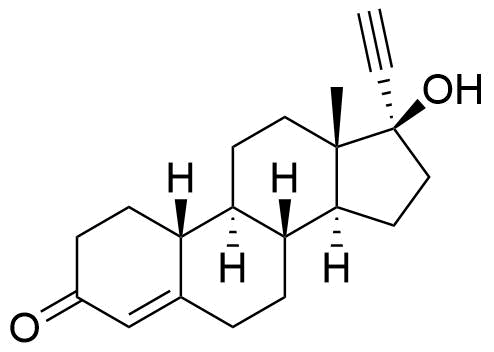
Norethindrone acetate, norethindrone enanthate, lynestrenol, and ethynodiol diacetate (and to a very small extent, norethynodrel) are prodrugs of norethindrone. Conversion of lynestrenol to ethynodiol to norethindrone facilitated by CYP2C9, CYP2C19, and CYP3A4. Conversion of norethynodrel to norethindrone is accomplished by either non-enzymatic or enzymatic ketosteroid isomerization.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Prescription
072723