Desogestrel Metabolites
| Name | Structure | Notes |
|---|---|---|
| 3-Keto-5α-dihydrodesogestrel |
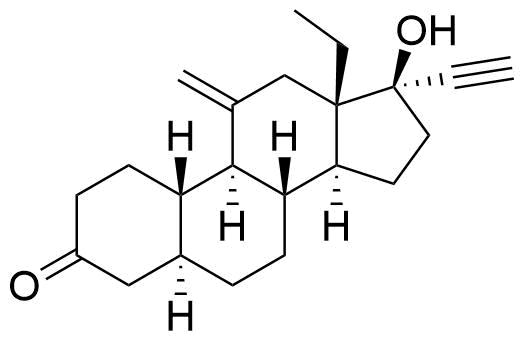
|
Lower Progesterone Receptor and Estrogen Receptor activity than etonogestrel. |
| Other Desogestrel Metabolites | Glucuronide and sulfate conjugates of desogestrel and some of its metabolites (3β-hydroxydesogestrel, 15β-hydroxydesogestrel) are also formed. | |
| 15β-Hydroxydesogestrel |
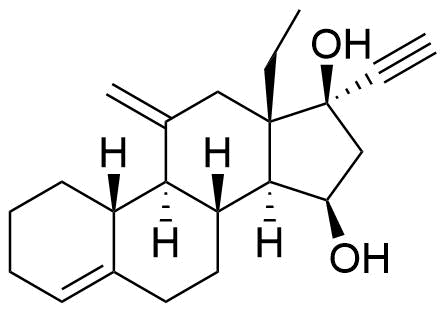
|
|
| 16-Hydroxydesogestrel |
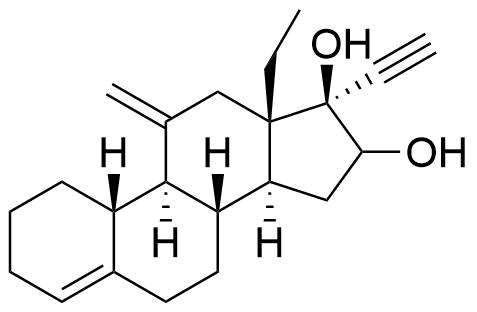
|
|
| 6α-Hydroxydesogestrel |
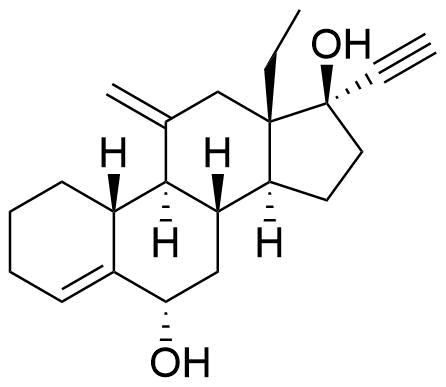
|
|
| 2-Hydroxydesogestrel |
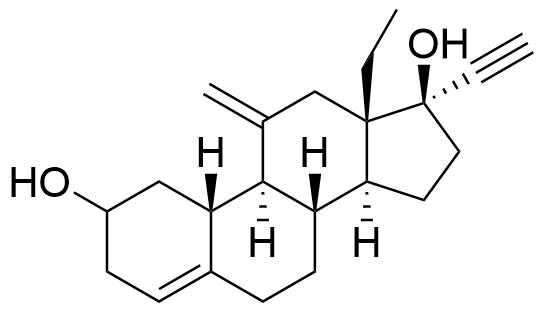
|
Pharmacologically inactive. |
| 3β,5α-Tetrahydrodesogestrel |
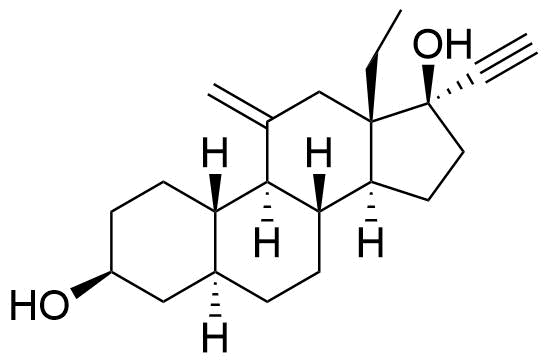
|
|
| 3α,5α-Tetrahydrodesogestrel |
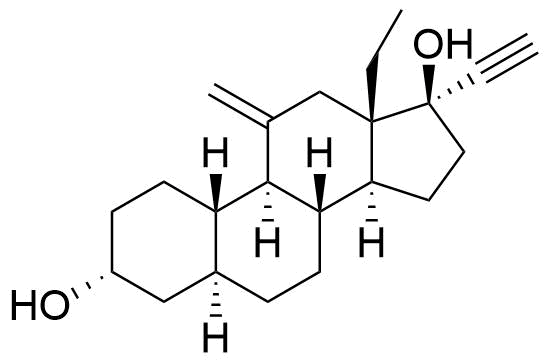
|
|
| 13-Hydroxyethyldesogestrel |
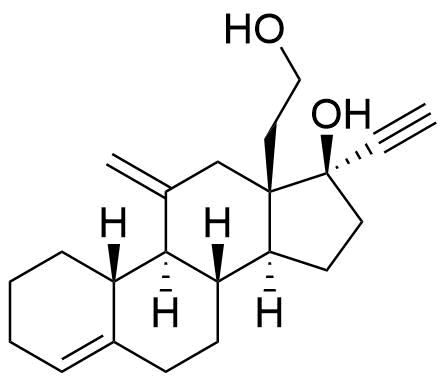
|
|
| 15α-Hydroxydesogestrel |
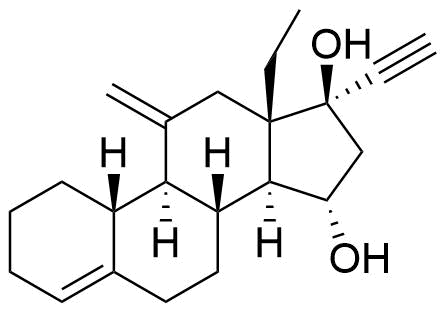
|
|
| 6β-Hydroxydesogestrel |
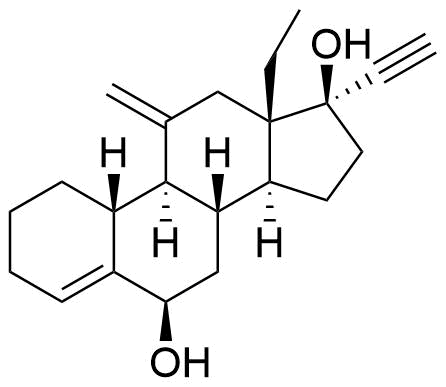
|
Catalyzed by CYP3A4. |
| 5α-Dihydrodesogestrel |
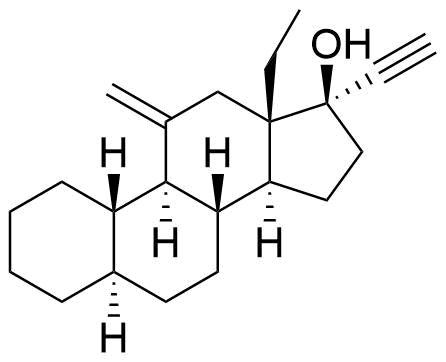
|
|
| 3β-Hydroxydesogestrel |
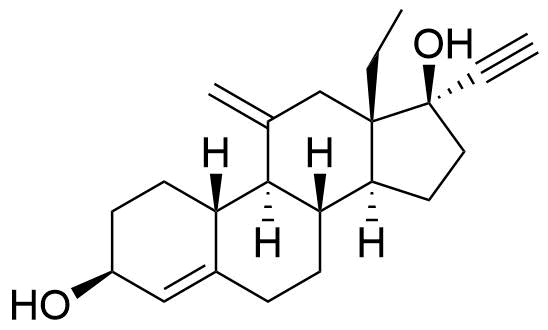
|
Minor metabolite. Rapidly oxidized to etonogestrel. Initial in vitro studies indicated that CYP2C9 and CYP2C19 were responsible for this conversion, however later human in vivo studies refuted this and indicated CYP3A4 (Korhonen et al. 2005). |
| 3α-Hydroxydesogestrel |
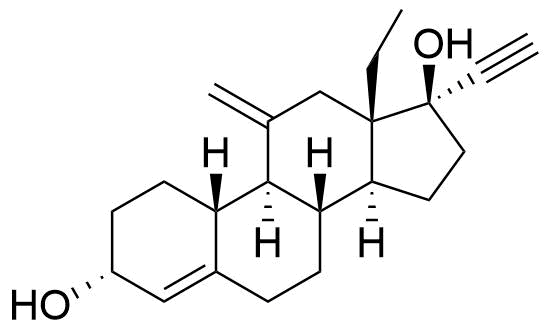
|
Primary metabolite. Rapidly oxidized to etonogestrel. Initial in vitro studies indicated that CYP2C9 and CYP2C19 were responsible for this conversion, however later human in vivo studies refuted this and indicated CYP3A4 (Korhonen et al. 2005). |
| Etonogestrel |
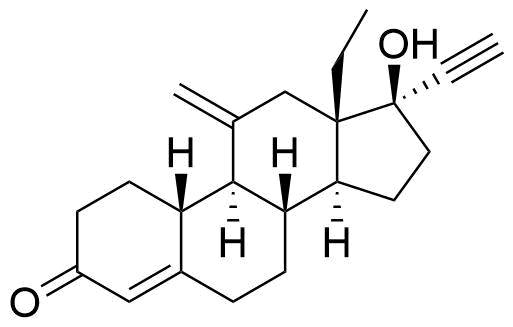
|
Primary metabolite of desogestrel, resulting from the rapid oxidation of desogestrel metabolites 3α- and 3β-hydroxydesogestrel by 3β-hydroxysteroid dehydrogenase/Delta 5-->4 isomerase type 1. |