Δ5(10)-8β-Levonorgestrel
Structure
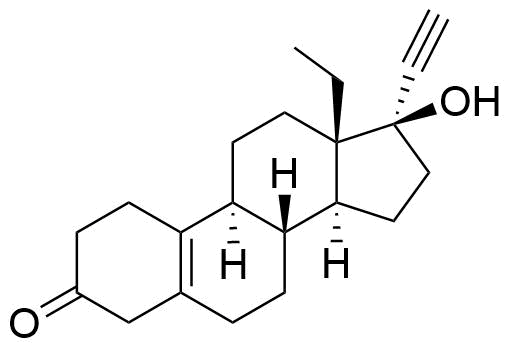
Other Names & Identifiers
- BP Levonorgestrel Impurity B
- 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-5(10)-en-20-yn-3-one
CASRN
Reaxys ID Number
2626494
References
Molecular Formula
C21H28O2
References
Molecular Weight
312.45 g/mol
References
Infrared Spectra Available
see references
References
- Smith HP, P. C.; Smith, L. L.; Gadsby, B.; Hartley, D.; Ledig, K.; Herbst, D.; Wendt, G. R.; Fisher, J.; Pattison, T. W.; Watson, D. H. P.; Siddall, J.; McLoughlin, B. J.; Tokolics, J.; Siuda, J.; Rees, R.; McMenamin, J.; Douglas, G. H.; Foell, T.; Edgren, R. A.; Buzby, G. C.; Jansen, A. B. A.; Hughes, G. A. Totally synthetic steroid hormones. 2. 13β-alkylgona-1,3,5(10)-trienes 13β-alkylgon-4-en-3-ones + related compounds. Journal of the Chemical Society. 1964:4472-&. (View all citations for this reference)
- Cessac, James W., Rao, Pemmaraju N., Blye Richard P., Kim, Hyun K., 11 beta-substituted 13 beta-ethyl gonane derivatives exhibit reversal of antiprogestational activity; Steroids 1998; 63 (50-57) (View all citations for this reference)
Structural Difference from API
no double bond between C4 & C5; additional double bond between C5 & C10
References
Source of Impurity
Synthetic side product formed during the Birch Reduction/hydrolysis
References
- Görög, S.; Babják, M.; Balogh, G.; Brlik, J.; Dravecz, F.; Gazdag, M.; Horváth, P.; Laukó, A.; Varga, K. Estimation of impurity profiles of drugs and related materials Part 19: Theme with variations. Identification of impurities in 3-oxosteroids. Journal of Pharmaceutical and Biomedical Analysis.1998, 18 (4), 511–525. 1998; 18:511-25. (View all citations for this reference)
Melting Point
169-171° C in acetonitile; water; 182-190° C in petroleum ether; ethyl acetate
References
- Online Subscription Database. Reed Elsevier Properties SA. (View all citations for this reference)
- Cessac, James W., Rao, Pemmaraju N., Blye Richard P., Kim, Hyun K., 11 beta-substituted 13 beta-ethyl gonane derivatives exhibit reversal of antiprogestational activity; Steroids 1998; 63 (50-57) (View all citations for this reference)
- Smith H, Phillips PC, Smith LL, Gadsby B, Hartley D, Ledig K, Herbst D, Wendt GR, Fisher J, Pattison TW, Watson DHP, Siddall J, McLoughlin BJ, Tokolics J, Siuda, J Rees R, McMenamin J, Douglas GH, Foell T, Edgren RA, Buzby GC, Jansen ABA, Hughes GA; Totally synthetic steroid hormones. 2. 13β-alkylgona-1,3,5(10)-trienes 13β-alkylgon-4-en-3-ones + related compounds. Journal of the Chemical Society, 1964 (4472-4481) (View all citations for this reference)
NMR Spectra Available
see references
References
- Cessac, James W., Rao, Pemmaraju N., Blye Richard P., Kim, Hyun K., 11 beta-substituted 13 beta-ethyl gonane derivatives exhibit reversal of antiprogestational activity; Steroids 1998; 63 (50-57) (View all citations for this reference)
Limits
0.3%
References
- British Pharmacopoeia 2017: Levonorgestrel monograph. (View all citations for this reference)