10β-Hydroxylevonorgestrel
Structure
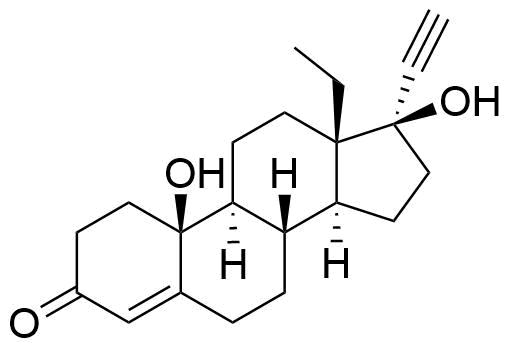
Other Names & Identifiers
- BP Levonorgestrel Impurity I
- 13-ethyl-10,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one
CASRN
21508-50-9
Molecular Formula
C21H28O3
References
Molecular Weight
328.54 g/mol
References
Source of Impurity
Formed through oxidation of final product and/or intermediates
library_books
References
- Gorog SB, M.; Csizer, E.; Dravetz, F.; Gazdag, M.; Herenyi, B. Estimation of impurity profiles of drugs and related materials .14. The role of HPLC/diode-array UV spectroscopy in the identification of minor components (impurities, degradation products, metabolites) in various matrices. Journal of Pharmaceutical and Biomedical Analysis. 1995; 14:85-92. (View all citations for this reference)
- Horvath PB, G.; Brlik, J.; Csehi, A.; Dravecz, F.; Halmos, Z.; Lauko, A.; Renyei, M.; Varga, K.; Gorog, S. Estimation of impurity profiles of drugs and related materials .16. Identification of the side-products of the ethinylation step in the synthesis of contraceptive gestogens. Journal of Pharmaceutical and Biomedical Analysis. 1997; 15:1343-9. (View all citations for this reference)
Limits
0.1%
References
- British Pharmacopoeia 2017: Levonorgestrel monograph. (View all citations for this reference)
- Berta, R.; Babjak, M.; Gazdag, M. A Study of Some Practical Aspects of High Temperature Liquid Chromatography in Pharmaceutical Applications. J. Pharm. Biomed. Anal. 2011, 54 (3), 458–462. (View all citations for this reference)