Methoxydienone
Structure
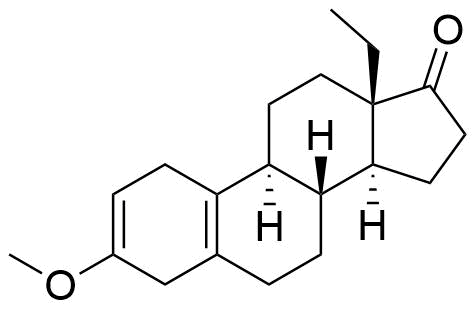
Other Names & Identifiers
- BP Levonorgestrel Impurity R
- 13-Ethyl-3-methoxygona-2,5(10)-dien-17-one
- Methoxygonadiene
CASRN
Notes
Anabolic steroid.
Molecular Formula
C20H28O2
References
Molecular Weight
300.44 g/mol
References
Reaxys ID Number
2996354
References
Source of Impurity
Synthetic side product formed during the ethinylation step
References
- Horvath PB, G.; Brlik, J.; Csehi, A.; Dravecz, F.; Halmos, Z.; Lauko, A.; Renyei, M.; Varga, K.; Gorog, S. Estimation of impurity profiles of drugs and related materials .16. Identification of the side-products of the ethinylation step in the synthesis of contraceptive gestogens. Journal of Pharmaceutical and Biomedical Analysis. 1997; 15:1343-9. (View all citations for this reference)
Melting Point
184-185° C in methanol, 152-160° C in methanol
References
- Rufer CK, H.; Schroder, E.; Kieslich, K.; Gibian, H. Totalsynthese optisch aktiver steroide. 3. Totalsynthese von optisch aktiven 13-athyl-gonan-derivaten. Annalen Der Chemie-Justus Liebig. 1967; 702:141-+. (View all citations for this reference)
- Smith HP, P. C.; Smith, L. L.; Gadsby, B.; Hartley, D.; Ledig, K.; Herbst, D.; Wendt, G. R.; Fisher, J.; Pattison, T. W.; Watson, D. H. P.; Siddall, J.; McLoughlin, B. J.; Tokolics, J.; Siuda, J.; Rees, R.; McMenamin, J.; Douglas, G. H.; Foell, T.; Edgren, R. A.; Buzby, G. C.; Jansen, A. B. A.; Hughes, G. A. Totally synthetic steroid hormones. 2. 13β-alkylgona-1,3,5(10)-trienes 13β-alkylgon-4-en-3-ones + related compounds. Journal of the Chemical Society. 1964:4472-&. (View all citations for this reference)
Structural Difference from API
no ethynyl group at C17; double bonds between C2 & C3 and C5 & C10 instead of between C4 & C5; methoxy group at C3 instead of carbonyl; carbonyl on C17 instead of hydroxy and ethynl
References
Circular Dichroism
270-320 nm in dioxane
References
- Baier HD, G.; Quinkert, G. TOTAL SYNTHESIS VON (-)-NORGESTREL. Helvetica Chimica Acta. 1985; 68:1054-68. (View all citations for this reference)
Optical Rotary Power
156.2 -158 at 589 nm, 20° C
References
- Baier HD, G.; Quinkert, G. TOTAL SYNTHESIS VON (-)-NORGESTREL. Helvetica Chimica Acta. 1985; 68:1054-68. (View all citations for this reference)
- Rufer CK, H.; Schroder, E.; Kieslich, K.; Gibian, H. Totalsynthese optisch aktiver steroide. 3. Totalsynthese von optisch aktiven 13-athyl-gonan-derivaten. Annalen Der Chemie-Justus Liebig. 1967; 702:141-+. (View all citations for this reference)
Spectra
NMR, IR
References
- Baier HD, G.; Quinkert, G. TOTAL SYNTHESIS VON (-)-NORGESTREL. Helvetica Chimica Acta. 1985; 68:1054-68. (View all citations for this reference)
- Smith H, Phillips PC, Smith LL, Gadsby B, Hartley D, Ledig K, Herbst D, Wendt GR, Fisher J, Pattison TW, Watson DHP, Siddall J, McLoughlin BJ, Tokolics J, Siuda, J Rees R, McMenamin J, Douglas GH, Foell T, Edgren RA, Buzby GC, Jansen ABA, Hughes GA; Totally synthetic steroid hormones. 2. 13β-alkylgona-1,3,5(10)-trienes 13β-alkylgon-4-en-3-ones + related compounds. Journal of the Chemical Society, 1964 (4472-4481) (View all citations for this reference)
Limits
0.1%
References
- British Pharmacopoeia 2017: Levonorgestrel monograph. (View all citations for this reference)
- Wikipedia: Methoxydienone. (View all citations for this reference)