13-Ethyl-3-methoxy-18,19-dinor-17α-pregna-1,3,5(10)-trien-20-yn-17-ol
Structure
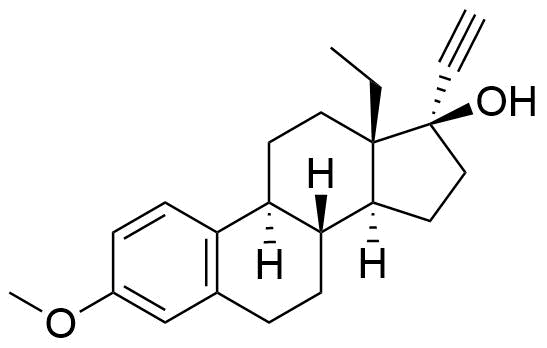
Other Names & Identifiers
- BP Levonorgestrel Impurity V
CASRN
14009-70-2
Reaxys ID Number
2626409
References
Chemical Name
3-methoxy, 17-α-ethinyl-13-ethyl-1,3,5(10) - gonatriene-17-ol
References
Molecular Formula
C22H28O2
References
Molecular Weight
324.46 g/mol
References
Structural Difference from API
methoxy at C3; no double bond between C4&C5, double bonds between C1&C2, C3&C4, C5&C10.
References
Melting Point
102-104° C, 150-160° C in aqueous methanol
library_books
References
- Hiraga K. Synthesis of Racemic and Optically Active 13β-Ethylgonanes. Chemical & Pharmaceutical Bulletin. 1965; 13:1289-&. (View all citations for this reference)
- Smith HP, P. C.; Smith, L. L.; Gadsby, B.; Hartley, D.; Ledig, K.; Herbst, D.; Wendt, G. R.; Fisher, J.; Pattison, T. W.; Watson, D. H. P.; Siddall, J.; McLoughlin, B. J.; Tokolics, J.; Siuda, J.; Rees, R.; McMenamin, J.; Douglas, G. H.; Foell, T.; Edgren, R. A.; Buzby, G. C.; Jansen, A. B. A.; Hughes, G. A. Totally synthetic steroid hormones. 2. 13β-alkylgona-1,3,5(10)-trienes 13β-alkylgon-4-en-3-ones + related compounds. Journal of the Chemical Society. 1964:4472-&. (View all citations for this reference)
Limits
0.15%
References
- Horvath PB, G.; Brlik, J.; Csehi, A.; Dravecz, F.; Halmos, Z.; Lauko, A.; Renyei, M.; Varga, K.; Gorog, S. Estimation of impurity profiles of drugs and related materials .16. Identification of the side-products of the ethinylation step in the synthesis of contraceptive gestogens. Journal of Pharmaceutical and Biomedical Analysis. 1997; 15:1343-9. (View all citations for this reference)
- British Pharmacopoeia 2017: Levonorgestrel monograph. (View all citations for this reference)