Drospirenone
Drospirenone (DRSP) is a synthetic progestogen used in combined oral contraceptives and hormone replacement therapy. Unlike other progestins, DRSP is a spirolactone, instead of a progesterone or testosterone derivative.
Tags
Approvals
US FDA-Approved
Identifiers
Abbreviation
DRSP
References
Names
1,2-dihydrospirorenone
CASRN
67392-87-4
References
PubChem CID
68873
ECHA InfoCard
- 100.060.599
- EC / List #: 266-679-2
IUPHAR/BPS
2874
DrugBank Accession Number
DB01395
References
- DrugBank: Drospirenone
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
N295J34A25
KEGG Entry Number
D03917
ChEBI ID
CHEBI:50838
ChEMBL ID
CHEMBL1509
ChemSpider ID
62105
Physical & Chemical Properties
Molecular Formula
C24H30O3
References
Molecular Weight
366.50 g/mol
References
Melting Point
USP: 198°-203° (dry over silica gel for at least 24 hours first)
IARC: 201.3 °C
References
- United State Pharmacopoeia 40 (2017): Drospirenone monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Drospirenone (View all citations for this reference)
- Wang, Q.; Sun, Q. M.; Li, S. S.; Guo, L. Q.; Li, H. X-Ray Powder Diffraction Data for Drospirenone, C24H30O3. Powder Diffr. 2016, 31 (1), 63–65. (View all citations for this reference)
logP
4.02
Specific Optical Rotation
USP: -187°-193° at 20° on anhydrous and solvent-free basis, 10 mg/mL in methanol
HSDB, IARC, Toxnet: -182° at 22° C/D (c = 0.5 in chloroform)
References
- Hazardous Substances Database: Drospirenone. (View all citations for this reference)
- United State Pharmacopoeia 40 (2017): Drospirenone monograph. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Drospirenone (View all citations for this reference)
Density
1.236 g/cm3
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
| Acute Oral Toxicity | 4 | H302 | Harmful if swallowed |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360 | May damage fertility or the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children |
Mutagenicity
Not mutagenic in a number of tests: Ames, Chinese Hamster Lung gene mutation, human lymphocytes, mouse micronucleus.
Genotoxicity
Not found to be genotoxic in human lymphocyte assay in vitro and mouse bone marrow micronucleus test in vivo.
References
- (1) Reimann, R.; Kalweit, S.; Lang, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. II. Communication: Examination for the Induction of Cytogenetic Damage Using the Chromosomal Aberration Assay on Human Lymphocytes in Vitro and the Mouse Bone Marrow Micronucleus Test in vivo. Environ. Mol. Mutagen. 1996, 28 (2), 133–144. (View all citations for this reference)
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist
Androgen Receptor Activity
Partial antagonist (about 30% activity of cyproterone acetate)
References
- Sitruk-Ware, R., New progestagens for contraceptive use. Hum. Reprod. Update 2006, 12 (2), 169-78. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Sitruk-Ware, R.; Nath, A., The use of newer progestins for contraception. Contraception 2010, 82 (5), 410-7. (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Krattenmacher, R. Drospirenone: Pharmacology and Pharmacokinetics of a Unique Progestogen. Contraception 2000, 62 (1), 29–38. (View all citations for this reference)
Estrogen Receptor Activity
Antagonist
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Glucocorticoid Receptor Activity
No activity
References
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Mineralocorticoid Receptor Activity
Antagonist (antimineralocorticoid or aldosterone antagonist). Agonist reports vary: "weak" (Africander) to "strong" (Bartsch).
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Bartsch, V., Gynaecological uses of dienogest alone and in combination with oestrogens. Journal of Medical Drug Reviews 2015, 5, 1-31. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Sitruk-Ware, R., New progestagens for contraceptive use. Hum. Reprod. Update 2006, 12 (2), 169-78. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Hatcher, Robert A. Contraceptive Technology. New York: Ardent Media, Inc, 2004. Print. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- Sitruk-Ware, R.; El-Etr, M., Progesterone and related progestins: potential new health benefits. Climacteric 2013, 16, 69-78. (View all citations for this reference)
- Wikipedia: Drospirenone (View all citations for this reference)
Bioavailability
76-85%
References
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Krattenmacher, R. Drospirenone: Pharmacology and Pharmacokinetics of a Unique Progestogen. Contraception 2000, 62 (1), 29–38. (View all citations for this reference)
- Toxnet: Drospirenone (View all citations for this reference)
Elimination Half-Life (t1/2)
25-33 h
References
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Stanczyk, F. Z., Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev. Endocr. Metab. Disord. 2002, 3 (3), 211-224. (View all citations for this reference)
- Krattenmacher, R. Drospirenone: Pharmacology and Pharmacokinetics of a Unique Progestogen. Contraception 2000, 62 (1), 29–38. (View all citations for this reference)
- Toxnet: Drospirenone (View all citations for this reference)
Serum Protein Binding
95-96% bound to serum albumin. No binding to SHBG or CBG.
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- Krattenmacher, R. Drospirenone: Pharmacology and Pharmacokinetics of a Unique Progestogen. Contraception 2000, 62 (1), 29–38. (View all citations for this reference)
- Toxnet: Drospirenone (View all citations for this reference)
Metabolism
At least 20 different metabolites observed in urine and feces. Metabolites generated independently of the cytochrome P450 system, with only minor metabolism by CYP3A4.
References
- Krattenmacher, R. Drospirenone: Pharmacology and Pharmacokinetics of a Unique Progestogen. Contraception 2000, 62 (1), 29–38. (View all citations for this reference)
- Nanda, K.; Stuart, G. S.; Robinson, J.; Gray, A. L.; Tepper, N. K.; Gaffield, M. E. Drug Interactions between Hormonal Contraceptives and Antiretrovirals. AIDS 2017, 31 (7), 917–952. (View all citations for this reference)
Excretion
Excreted mostly as glucuronide and sulfate conjugates in urine and feces.
Cmax
60-87 ng/mL
Tmax
1-2 h
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- Su, Y.; Lian, Q. Q.; Ge, R. S., Contraceptives with novel benefits. Expert Opin Investig Drugs 2012, 21 (1), 83-90. (View all citations for this reference)
- Krattenmacher, R. Drospirenone: Pharmacology and Pharmacokinetics of a Unique Progestogen. Contraception 2000, 62 (1), 29–38. (View all citations for this reference)
Enzyme Interactions
Inhibits 3β-hydroxysteroid dehydrogenase type 2 (HSD)
References
- Toit, R. L. D.; Perkins, M. S.; Snoep, J. L.; Storbeck, K. H.; Africander, D., Fourth-generation progestins inhibit 3β-hydroxysteroid dehydrogenase type 2 and modulate the biosynthesis of endogenous steroids. PLoS ONE 2016, 11, 1-24. (View all citations for this reference)
Metabolites
Name
Structure
Notes
At least 20 different metabolites have been found, many with sulfate and glucuronide conjugates.
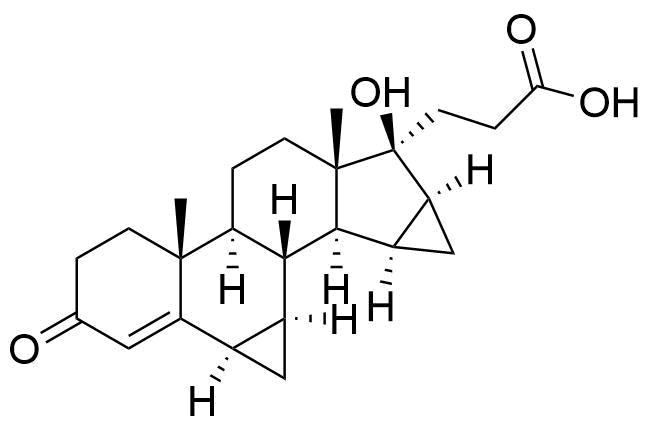
One of 2 major plasma metabolites. Formed independently of the cytochrome P450 enzyme system.
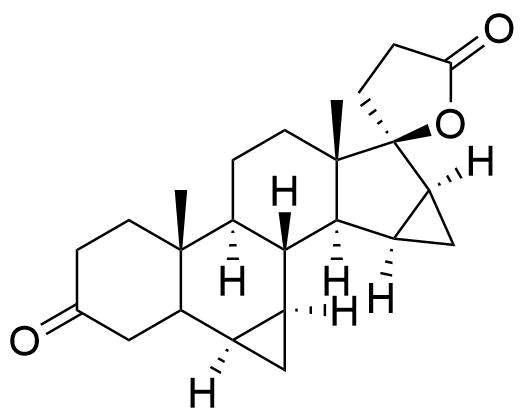
One of 2 major plasma metabolites. Formed independently of the cytochrome P450 enzyme system. Circulates as the sulfate conjugate.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
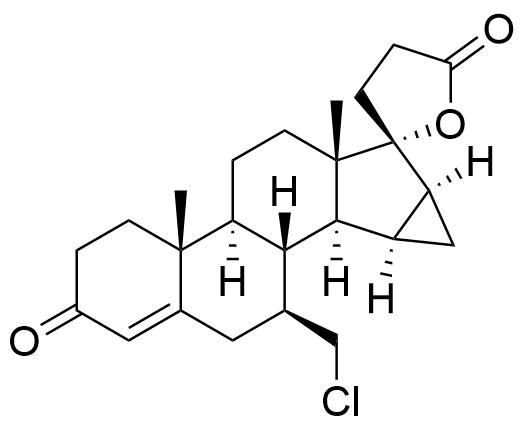
932388-89-1
- BP Drospirenone Impurity H
- 17-Hydroxy-7β-chloromethyl-15β,16β-methylene-3-oxo-17β-pregn-4-ene-21-carboxylic acid, γ-lactone
- 3'-chloro-3',6-seco-17-epidrospirenone
- 7β-(chloromethyl)-3-oxo-15α,16α-dihydro-3'H-cyclopropa[15,16]pregn-4-ene-21,17-carbolactone
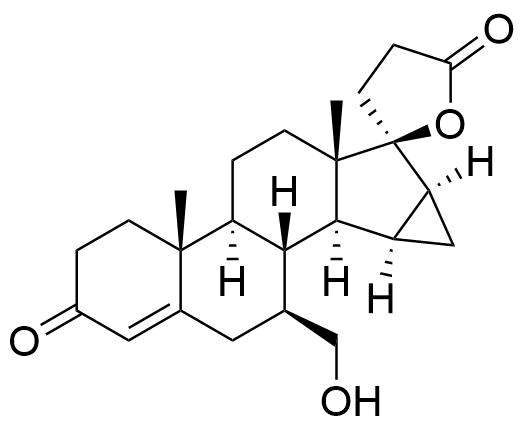
- BP Drospirenone Impurity B
- 17-hydroxy-7β-hydroxymethyl-15β,16β-methylene-3-oxo-17α-pregn-4-ene-21-carboxylic acid, γ-lactone
- 7β-hydroxymethyl drospirenone derivative
- 7β-(hydroxymethyl)-3-oxo-15α,16α-dihydro-3'H-cyclopropa[15,16]-17α-pregn-4-ene-21,17-carbolactone
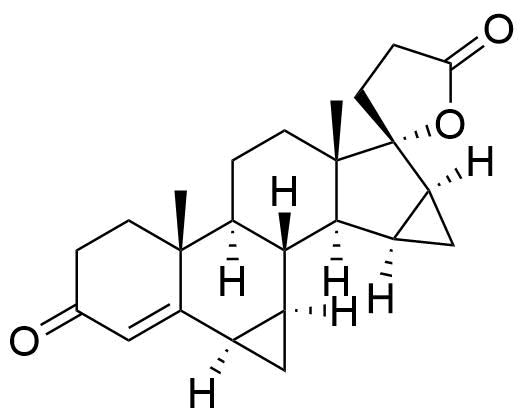
90457-65-1
- USP Drospirenone Related Compound A
- BP Drospirenone Impurity E
- 17-Hydroxy-6β,7β:15β,16β-dimethylene-3-oxo-17β-pregn-4-ene-21-carboxylic acid, γ-lactone
- 3-oxo-6α,7α,15α,16α-tetrahydro-3'H,3''H-dicyclopropa[6,7:15,16]pregn-4-ene-21,17-carbolactone
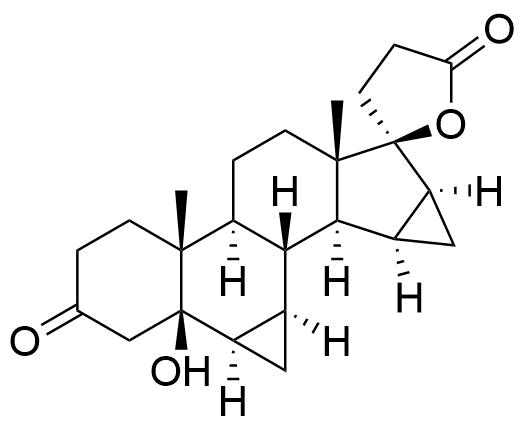
197721-70-3
- 5β,17-Dihydroxy-6β,7β:15β,16β-dimethylene-3-oxo-17α-pregnan-211-carboxylic acid, γ-lactone
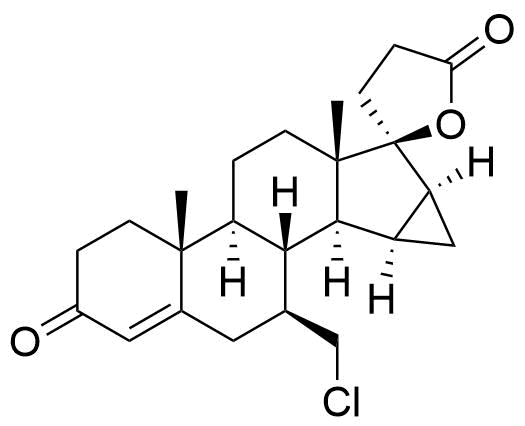
932388-90-4
- BP Drospirenone Impurity G
- 17-Hydroxy-7β-chloromethyl-15β,16β-methylene-3-oxo-17α-pregn-4-ene-21-carboxylic acid, γ-lactone
- 3'-chloro-3',6-secodrospirenone
- 7β-(chloromethyl)-3-oxo-15α16α-dihydro-3'H-cyclopropa[15,16]-17α-pregn-4-ene-21,17-carbolactone
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Prescription
022574