3-Deoxolevonorgestrel
Structure
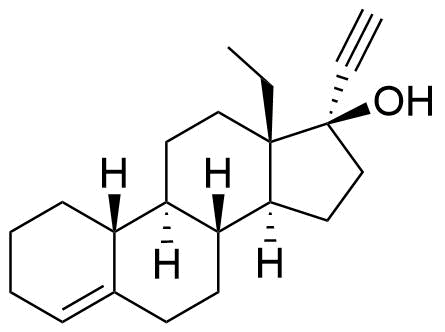
Other Names & Identifiers
- BP Levonorgestrel Impurity D
- 13-ethyl-18,19-dinor-17α-pregn-4-en-20-yn-17-ol
- 17α-ethinyl-13-ethyl-4-gonene-17-ol
CASRN
Reaxys ID Number
2565263
References
Molecular Formula
C21H30O
References
Structural Difference from API
missing carbonyl at C3
References
Source of Impurity
Synthetic side product formed during the Birch reduction steporiginating from a side product of the Birch reduction step in the synthesis of norgestrel (ref = Hovrath, 1997, Gorog 1998); over-reduction of phenolic ring A in the Birch reduction step of the synthesis (ref = Gorog, 1998)
References
- Görög, S.; Babják, M.; Balogh, G.; Brlik, J.; Dravecz, F.; Gazdag, M.; Horváth, P.; Laukó, A.; Varga, K. Estimation of impurity profiles of drugs and related materials Part 19: Theme with variations. Identification of impurities in 3-oxosteroids. Journal of Pharmaceutical and Biomedical Analysis.1998, 18 (4), 511–525. 1998; 18:511-25. (View all citations for this reference)
- Horvath PB, G.; Brlik, J.; Csehi, A.; Dravecz, F.; Halmos, Z.; Lauko, A.; Renyei, M.; Varga, K.; Gorog, S. Estimation of impurity profiles of drugs and related materials .16. Identification of the side-products of the ethinylation step in the synthesis of contraceptive gestogens. Journal of Pharmaceutical and Biomedical Analysis. 1997; 15:1343-9. (View all citations for this reference)
Molecular Weight
298.47 g/mol
References
Melting Point
107-108° C in methanol
References
- Online Subscription Database. Reed Elsevier Properties SA. (View all citations for this reference)
- Smith HP, P. C.; Smith, L. L.; Gadsby, B.; Hartley, D.; Ledig, K.; Herbst, D.; Wendt, G. R.; Fisher, J.; Pattison, T. W.; Watson, D. H. P.; Siddall, J.; McLoughlin, B. J.; Tokolics, J.; Siuda, J.; Rees, R.; McMenamin, J.; Douglas, G. H.; Foell, T.; Edgren, R. A.; Buzby, G. C.; Jansen, A. B. A.; Hughes, G. A. Totally synthetic steroid hormones. 2. 13β-alkylgona-1,3,5(10)-trienes 13β-alkylgon-4-en-3-ones + related compounds. Journal of the Chemical Society. 1964:4472-&. (View all citations for this reference)
Pub Chem ID
29979853
References
Limits
0.1%
References
- British Pharmacopoeia 2017: Levonorgestrel monograph. (View all citations for this reference)