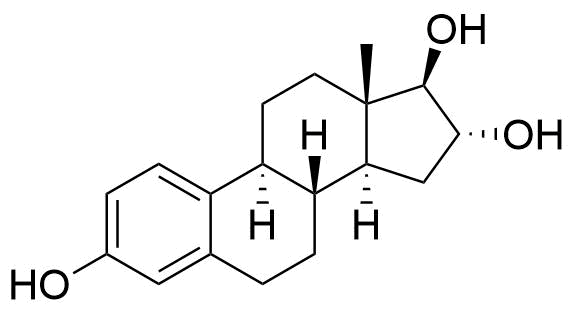
Estriol
Structure
CASRN
50-27-1
Source
Formed from 16a-hydroxylation and 17-keto reduction of estrone.
Notes
Major urinary metabolite. Formed from the estradiol metabolite estrone. 80 times less potent estrogen agonist than estradiol.
References
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Toxnet: Estradiol. (View all citations for this reference)