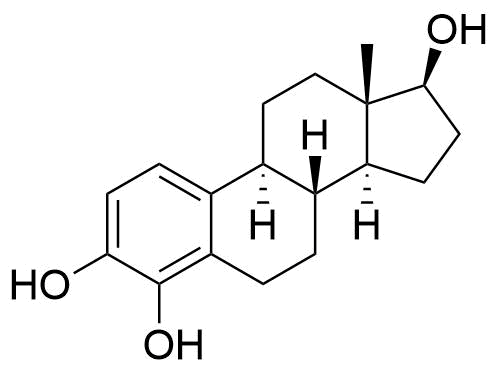
| 4-Hydroxyestradiol |
|
Formed by CYP1B1 in the liver. Minor pathway in liver but is formed in larger amount in extrahepatic tissues. Thought to be the most carcinogenic of all estradiol metabolites. Can bind to estrogen receptors. |
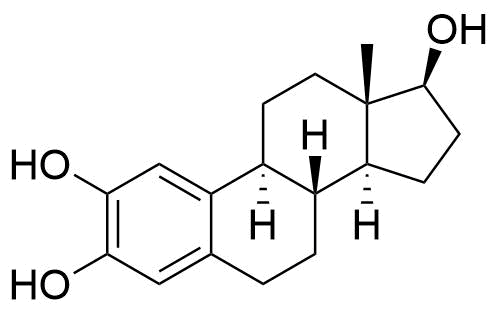
| 2-Hydroxyestradiol |
|
Formed in liver by CYP1A2, CYP3A4, and CYP2C9. Major metabolic pathway of estradiol in liver. Has about 7% and 11% the affinity of estradiol to ERα and ERβ, respectively. Weakly estrogenic, with some antagonistic effects. Can be further metabolized to 2-methoxyestradiol by catechol O-methyltransferase (COMT) in the liver. Can also be metabolized to free radicals that cause DNA damage. |
| Other Estradiol Metabolites |
|
A number of other hydroxylated metabolites are also formed (6α-, 6β-, 7α-, 12β-, 15α-, 15β-, 16α, and 16β-hydroxyestradiol). Estradiol may also be metabolized to hydroxyestrones, such as 2-, 4-, and 16α-hydroxyestrone. |
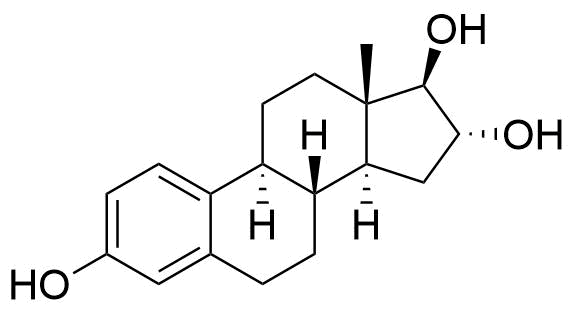
| Estriol |
|
Major urinary metabolite. Formed from the estradiol metabolite estrone. 80 times less potent estrogen agonist than estradiol. |
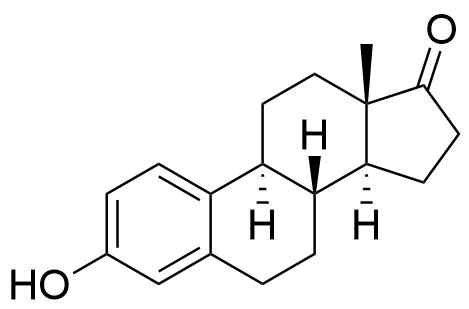
| Estrone |
|
Formed in the liver by CYP2C9, CYP2C19, and CYP2C8. Undergoes further conversion to estriol, the major urinary metabolite. 10 times less potent estrogen agonist that estradiol. |



