16β-Hydroxylevonorgestrel
Structure
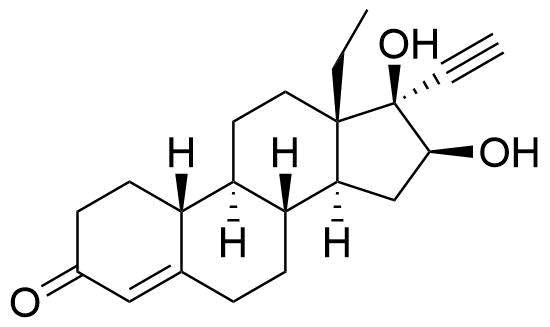
Source
Hydroxylation at C16.
Notes
Sulfate conjugate also present, as well as the sulfate conjugate of the 16α stereoisomer. Metabolite of both levonorgestrel and norgestrel.
References
- Stanczyk FZ. All progestins are not created equal. Steroids. 2003; 68:879-90. (View all citations for this reference)
- Stanczyk FZR, S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. 1990; 42:67-96. (View all citations for this reference)
- PubChem: Levonorgestrel. (View all citations for this reference)
- Besse, J. P.; Garric, J., Progestagens for human use, exposure and hazard assessment for the aquatic environment. Environ. Pollut. 2009, 157 (12), 3485-3494. (View all citations for this reference)
- Sisenwine, S. F.; Kimmel, H. B.; Liu, A. L.; Ruelius, H. W. The Presence of DL- , D- and L- Norgestrel and Their Metabolites in the Plasma of Women. Contraception 1975, 12 (3), 339–353. (View all citations for this reference)