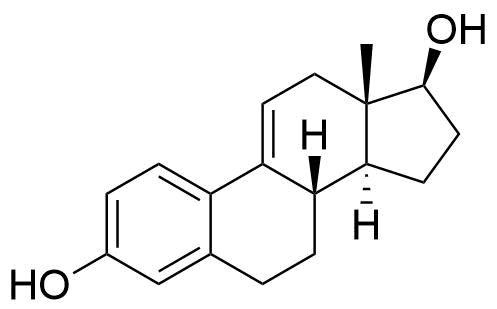
Estradiol
Estradiol (E2) is a natural estrogen and the primary female sex hormone. E2 and its esters, including estradiol cypionate and estradiol valerate, are used in combined oral contraceptives alongside progestins and as components of hormone replacement therapy.
Tags
Approvals
US FDA-ApprovedRelated Compounds
Estradiol Cypionate Estradiol Valerate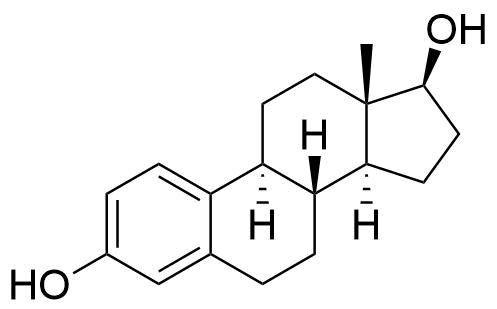
Identifiers
Abbreviation
E2
References
Names
- oestradiol
- 17β-oestradiol
- 17β-estradiol
- estra-1,3,5(10)-triene-3,17β-diol
References
CASRN
50-28-2
References
PubChem CID
5757
ECHA InfoCard
- 100.000.022
- EC / List #: 200-023-8
IUPHAR/BPS
1013
DrugBank Accession Number
DB00783
References
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
4TI98Z838E
KEGG Entry Number
D00105
Wikipedia Entry Name
Estradiol
ChEBI ID
CHEBI:16469
ChemSpider ID
5554
NIST
Estradiol
ATC Code(s)
- G03FB12
- G03FA12
- G03FA17
- G03FA16
- G03FA11
- G03FA14
- G03EA02
- G03AB08
- G03FB01
- G03FB09
- G03FA05
- G03AA14
- G03EA03
- G03FA08
- G03FB05
- G03FB11
- G03HB01
- G03FB07
- G03FA07
- G03CA53
- G03FB04
- G03CA03
- G03FA15
- G03FB10
- G03FA06
- G03EA01
- G03FB02
- G03FA09
- G03FA13
- G03FA01
- G03FA10
- G03FA04
- G03FB08
- G02BB01
- G03FA03
- G03FB03
- G03FA02
- G03FB06
References
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Physical & Chemical Properties
Molecular Formula
C18H24O2
References
Molecular Weight
272.38 g/mol
References
Melting Point
178.5° C (ChemIDPlus, Toxnet)
151-152° C (DrugBank)
References
- ChemIDPlus: A Toxnet Database. Estradiol. (View all citations for this reference)
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Toxnet: Estradiol. (View all citations for this reference)
Solubility
3.9 mg/L water at 27° C. Freely soluble in alcohol,;soluble in acetone, dioxane, other organic solvents; sparingly soluble in vegetable oils.
logP
4.01
References
- ChemIDPlus: A Toxnet Database. Estradiol. (View all citations for this reference)
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Toxnet: Estradiol. (View all citations for this reference)
Specific Optical Rotation
+76 - +83° at 25 °C for D (sodium) line (dioxane)
UV Max Absorption
225, 280 nm
Storage Conditions
Store between 15 and 30 °C.
References
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360 | May damage fertility or the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children | |
| Reproductive Toxicity | 1B | H360 | May damage fertility or the unborn child |
| Specific Target Organ Toxicity, Repeated Exposure | 1 | H372 | Causes damage to organs through prolonged or repeated exposure |
| Specific Target Organ Toxicity, Repeated Exposure | 2 | H373 | Causes damage to organs through prolonged or repeated exposure |
Side Effects
Respiratory tract infection, localized exfoliation, headache, endometrial disorder, vulvovaginal mucotic infection, vulvovaginal pruritus, application site reaction, breast tenderness
Carcinogenicity
"There is sufficient evidence in humans for the carcinogenicity of post-menopausal estrogen therapy. There is sufficient evidence in experimental animals for the carcinogenicity of estradiol and estrone."
References
- Toxnet: Estradiol. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
Mutagenicity
Not found to be mutagenic in the Ames Salmonella/microsome direct plate incorporation protocol.
References
- Lang, R.; Reimann, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. I. Communication: Examination for the Induction of Gene Mutations Using the Ames Salmonella/microsome Test and the HGPRT Test in V79 Cells. Environ. Mol. Mutagen. 1993, 21 (3), 272–304. (View all citations for this reference)
LD50
LD, rat subcutaneous: > 300 mg/kg
MRTD
0.5 mg/kg/day
Biochemistry & Pharmacology
Estrogen Receptor Activity
Agonist
References
- Alam, S. M.; Pal, R.; Nagar, S.; Islam, M. A.; Saha, A., Pharmacophore search for anti-fertility and estrogenic potencies of estrogen analogs. J. Mol. Model. 2008, 14 (11), 1071-1082. (View all citations for this reference)
- KEGG: Estradiol (View all citations for this reference)
Target Pathways
Bioavailability
< 5%
References
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
Elimination Half-Life (t1/2)
Oral: 13-30 h (Stanczyk)
36 h (DrugBank)
20.1 h from oral administration (Toxnet)
References
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference) - Toxnet: Estradiol. (View all citations for this reference)
Serum Protein Binding
60% to albumin, 38% to SHBG.
References
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- Toxnet: Estradiol. (View all citations for this reference)
Metabolism
Hepatic, if oral administration.
Excretion
54% in urine, 6% in feces.
References
- Stanczyk, F. Z.; Archer, D. F.; Bhavnani, B. R., Ethinyl estradiol and 17 beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87 (6), 706-727. (View all citations for this reference)
- Toxnet: Estradiol. (View all citations for this reference)
Caco-2 Permeability
-4.77
References
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Indications
For the treatment of urogenital symptoms associated with post-menopausal atrophy of the vagina (such as dryness, burning, pruritus and dyspareunia) and/or the lower urinary tract (urinary urgency and dysuria).
References
- DrugBank: Estradiol
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
Metabolites
Name
Structure
Notes
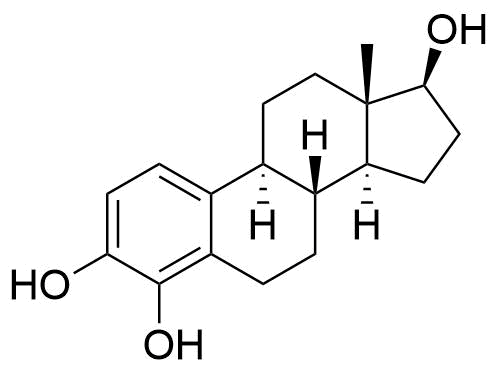
Formed by CYP1B1 in the liver. Minor pathway in liver but is formed in larger amount in extrahepatic tissues. Thought to be the most carcinogenic of all estradiol metabolites. Can bind to estrogen receptors.
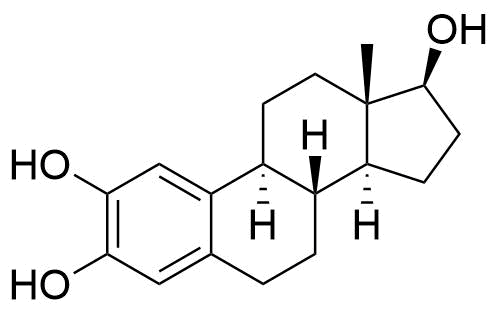
Formed in liver by CYP1A2, CYP3A4, and CYP2C9. Major metabolic pathway of estradiol in liver. Has about 7% and 11% the affinity of estradiol to ERα and ERβ, respectively. Weakly estrogenic, with some antagonistic effects. Can be further metabolized to 2-methoxyestradiol by catechol O-methyltransferase (COMT) in the liver. Can also be metabolized to free radicals that cause DNA damage.
A number of other hydroxylated metabolites are also formed (6α-, 6β-, 7α-, 12β-, 15α-, 15β-, 16α, and 16β-hydroxyestradiol). Estradiol may also be metabolized to hydroxyestrones, such as 2-, 4-, and 16α-hydroxyestrone.
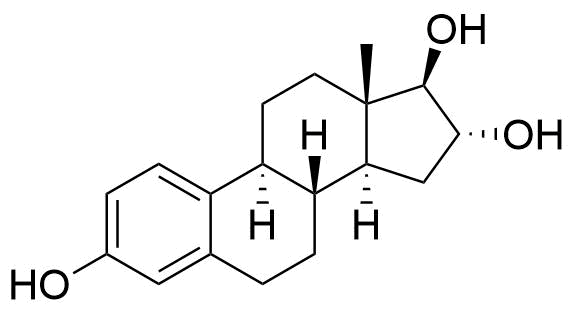
Major urinary metabolite. Formed from the estradiol metabolite estrone. 80 times less potent estrogen agonist than estradiol.
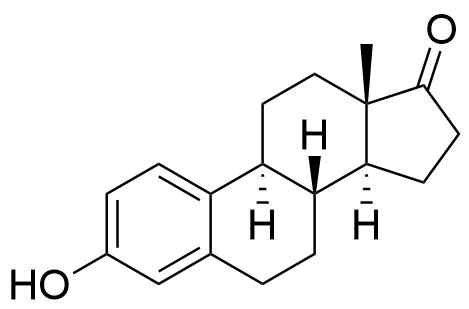
Formed in the liver by CYP2C9, CYP2C19, and CYP2C8. Undergoes further conversion to estriol, the major urinary metabolite. 10 times less potent estrogen agonist that estradiol.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
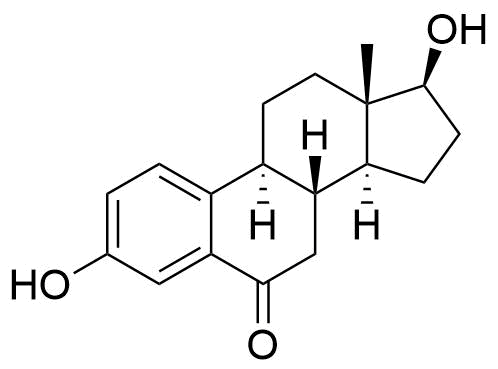
571-92-6
- BP Estradiol Vaginal Tablets Impurity 2
- 3,17β-dihydroxyestra-1,3,5(10)-trien-6-one
US FDA-Approved Products
Name
Formulation
Status
ANDA #
Extended Release Film
Prescription
020375
Prescription
020538
Discontinued; Prescription
021167
Discontinued
020323