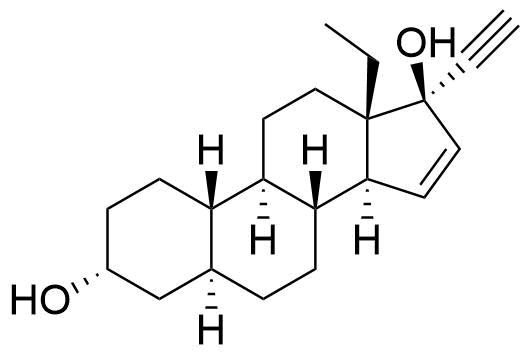
Gestodene
Gestodene (GSD) is a synthetic progestogen used in combined oral contraceptives. It is available in Europe but not the US.
Tags
Identifiers
Abbreviation
GSD
References
Names
- Δ15-norgestrel
- 15-dehydronorgestrel
- 17α-ethynyl-18-methylestra-4,15-dien-17β-ol-3-one
References
CASRN
60282-87-3
References
PubChem CID
3033968
ECHA InfoCard
100.056.478
DrugBank Accession Number
DB06730
References
- DrugBank: Gestodene
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8. doi: 10.1093/nar/gkx1037 (View all citations for this reference)
UNII
1664P6E6MI
KEGG Entry Number
D04316
Wikipedia Entry Name
Gestodene
ChEBI ID
CHEBI:135323
ChEMBL ID
CHEMBL1213583
ChemSpider ID
2298532
Physical & Chemical Properties
Molecular Formula
C21H26O2
References
Molecular Weight
310.44 g/mol
References
Appearance
Crystals
Melting Point
197.9° C
References
- ChemIDPlus: A Toxnet Database. Gestodene. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- Toxnet: Gestodene. (View all citations for this reference)
Solubility
8.11 mg/L water at 25 °C
Specific Optical Rotation
-185.7 (for the sodium D line)
Toxicology
GHS Hazard Code(s)
| Class | Category | Code | Description |
|---|---|---|---|
| Reproductive Toxicity | 2 | H361 | Suspected of damaging fertility or the unborn child |
| Carcinogenicity | 2 | H351 | Suspected of causing cancer if inhaled |
| Reproductive Toxicity | 1A | H360 | May damage fertility or the unborn child |
| Reproductive Toxicity, Effects On or Via Lactation | H362 | May cause harm to breast-fed children |
Side Effects
Headaches, breast tension, acne, nervousness, depression, dizziness
References
- Stanczyk, F. Z.; Archer, D. F., Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception 2014, 89 (4), 242-52. (View all citations for this reference)
Mutagenicity
Not found to be mutagenic in the Ames Salmonella/microsome direct plate incorporation protocol.
References
- Lang, R.; Reimann, R. Studies for a Genotoxic Potential of Some Endogenous and Exogenous Sex Steroids. I. Communication: Examination for the Induction of Gene Mutations Using the Ames Salmonella/microsome Test and the HGPRT Test in V79 Cells. Environ. Mol. Mutagen. 1993, 21 (3), 272–304. (View all citations for this reference)
LD50
mouse oral: 6 g/kg
Safety Profile Overview
Oral contraceptives containing gestodene are considered a safe, effective, well-tolerated option.
References
- Stanczyk, F. Z.; Archer, D. F., Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception 2014, 89 (4), 242-52. (View all citations for this reference)
Biochemistry & Pharmacology
Progesterone Receptor Activity
Agonist
References
Androgen Receptor Activity
Reported as agonist with high relative binding affinity and no antagonist activity, but Stanczyk notes that data consistent with low androgenic potential and strong antagonist effects demonstrated in vitro.
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Stanczyk, F. Z.; Archer, D. F., Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception 2014, 89 (4), 242-52. (View all citations for this reference)
Estrogen Receptor Activity
Antagonist
References
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
Glucocorticoid Receptor Activity
Reported as "weak" (Africander, Kuhl) and "active" (Ruan, Lello) agonist
References
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of dienogest. Maturitas 2012, 71 (4), 337-44. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
Mineralocorticoid Receptor Activity
Binds. Activity reports vary slightly: reported as an antagonist (Ruan, Lello, Kuhl), while Africander reports that some antagonist activity is seen in rat models and Stanczyk claims it to be relatively weak compared to progesterone.
References
- Africander, D.; Verhoog, N.; Hapgood, J. P., Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76 (7), 636-52. (View all citations for this reference)
- Kuhl, H., Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3-63. (View all citations for this reference)
- Stanczyk, F. Z.; Archer, D. F., Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception 2014, 89 (4), 242-52. (View all citations for this reference)
- Lello, S., Nomegestrol Acetate Pharmacology, Safety Profile and Therapeutic Efficacy. Drugs 2010, 70 (5), 541-559. (View all citations for this reference)
- Ruan, X.; Seeger, H.; Mueck, A. O., The pharmacology of nomegestrol acetate. Maturitas 2012, 71 (4), 345-53. (View all citations for this reference)
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Bioavailability
99%
References
- Schindler, A. E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J. R.; Schweppe, K. W.; Thijssen, J. H. H., Classification and pharmacology of progestins. Maturitas 2003, 46, 7-16. (View all citations for this reference)
- Stanczyk, F. Z., Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev. Endocr. Metab. Disord. 2002, 3 (3), 211-224. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- PubChem: Gestodene (View all citations for this reference)
- Toxnet: Gestodene. (View all citations for this reference)
Elimination Half-Life (t1/2)
12-14 h, 16-18 h
References
- Stanczyk, F. Z., Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev. Endocr. Metab. Disord. 2002, 3 (3), 211-224. (View all citations for this reference)
- Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e, 2011 > Estrogens and Progestins. Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann. (View all citations for this reference)
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- PubChem: Gestodene (View all citations for this reference)
- Toxnet: Gestodene. (View all citations for this reference)
Serum Protein Binding
75% to SHBG, 24% to albumin, 0.6% free (exact percentages vary depending on treatment)
References
- WHO International Agency for Research on Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 91: Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. 2007, Lyon, France. (View all citations for this reference)
- PubChem: Gestodene (View all citations for this reference)
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
- Toxnet: Gestodene. (View all citations for this reference)
Metabolism
Hepatic, primarily by CYP3A4
References
- PubChem: Gestodene (View all citations for this reference)
- Toxnet: Gestodene. (View all citations for this reference)
- Nanda, K.; Stuart, G. S.; Robinson, J.; Gray, A. L.; Tepper, N. K.; Gaffield, M. E. Drug Interactions between Hormonal Contraceptives and Antiretrovirals. AIDS 2017, 31 (7), 917–952. (View all citations for this reference)
Excretion
Only 1% excreted unchanged in urine.
References
- Besse, J. P.; Garric, J., Progestagens for human use, exposure and hazard assessment for the aquatic environment. Environ. Pollut. 2009, 157 (12), 3485-3494. (View all citations for this reference)
Apparent Volume of Distribution
0.7 L/kg
Cmax
5.6 ng/L from 0.1 mg gestodene/0.03 mg ethinyl estradiol tablet administered orally.
1.0 ng/mL from 0.025 mg gestodene administered orally.
3.6 ng/mL from 0.075 mg gestodene administered orally.
7.0 ng/mL from 0.125 mg gestodene administered orally.
Tmax
0.5 h from 0.1 mg gestodene/0.03 mg ethinyl estradiol tablet administered orally.
1.4-1.9 h for oral administration of 0.025, 0.075, or 0.125 mg gestodene tablets.
Clearance
0.8 mL/min/kg
Enzyme Interactions
- CYP3A4: inducer (PubChem, Toxnet), inhibitor (KEGG, Laine et al.)
- CYP3A7, CYP3A5, CYP2C19: inhibitor
References
- KEGG: Gestodene (View all citations for this reference)
- PubChem: Gestodene (View all citations for this reference)
- Laine, K.; Yasar, U.; Widen, J.; Tybring, G. A Screening Study on the Liability of Eight Different Female Sex Steroids to Inhibit CYP2C9, 2C19 and 3A4 Activities in Human Liver Microsomes. Pharmacol. Toxicol. 2003, 93 (2), 77–81. (View all citations for this reference)
- Toxnet: Gestodene. (View all citations for this reference)
Inhibition of Ovulation
0.04 mg/day
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Transformation of Endometrium
2-3 mg/cycle
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Menstrual Delay
~0.2 mg/day
References
- Rebar, R. W.; Zeserson, K., CHARACTERISTICS OF THE NEW PROGESTOGENS IN COMBINATION ORAL-CONTRACEPTIVES. Contraception 1991, 44 (1), 1-10. (View all citations for this reference)
Metabolites
Name
Structure
Notes
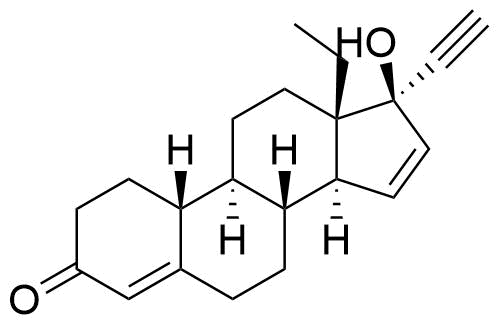
Significantly reduced progestational activity compared to gestodene. Selectively activates ERα.
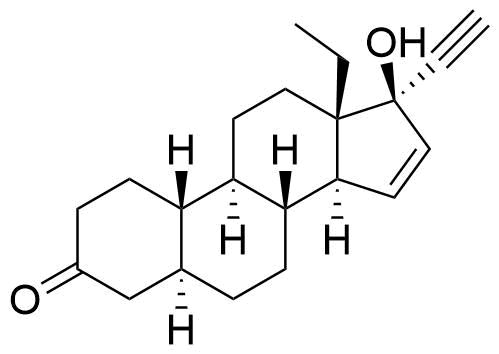
Significantly reduced progestational activity compared to gestodene. Selectively activates ERα.
Impurities
Name
Structure
CASRN
Other Names & Identifiers
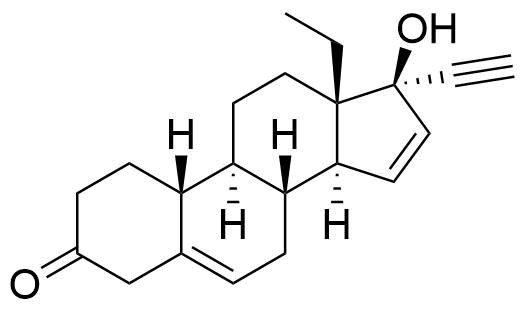
- BP Gestodene Impurity L
- 13-ethyl-17-hydroxy-18,19-dinor-17α-pregna-5,15-dien-20-yn-3-one
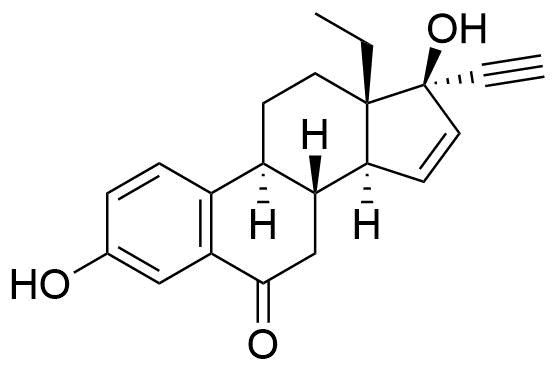
- BP Gestodene Impurity K
- 13-ethyl-3,17-dihydroxy-18,19-dinor-17α-pregna-1,3,5(10),15-tetraen-20-yn-6-one
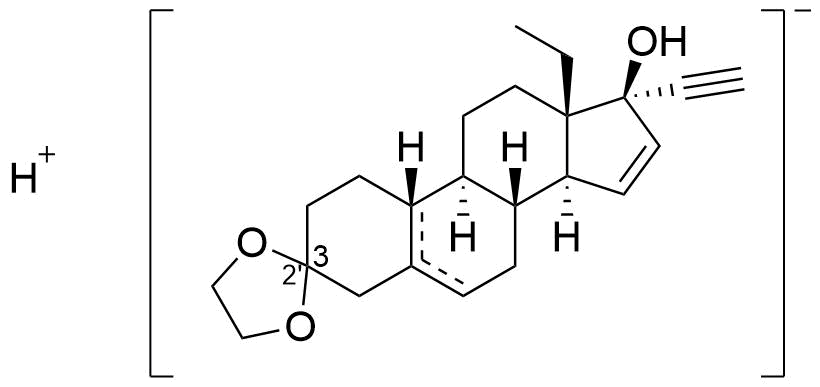
- BP Gestodene Impurity J
- 13-ethylspiro(18,19-dinor-17α-pregna-5,15-dien-20-yne-3,2'-[1,3]dioxolan)-17-ol and 13-ethylspiro(18,19-dinor-17α-pregna-5(10),15-dien-20-yne-3,2'-[1,3]dioxolan)-17-ol
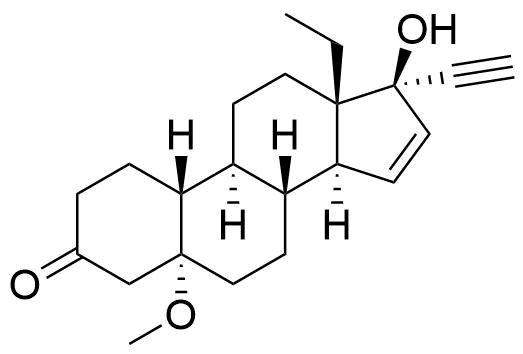
- BP Gestodene Impurity I
- 13-ethyl-17-hydroxy-5-methoxy-18,19-dinor-5α,17α-pregn-15-en-20-yn-3-one
- BP Gestodene Impurity H
- 13-ethyl-3-ethynyl-18,19-dinor-17α-pregna-3,5,15-trien-20-yn-17-ol